How to calculate the pH of 0.010 molarity acetic acid solution, if its dissociation constant is 1.8*10^-5 - Quora
What volume of a 2.5 M stock solution of acetic acid is required to prepare 100.0 milliliters of a .50 M acetic acid solution? | Socratic

acid, hydrochloric acid, and citric acid. Dilution factor is calculated... | Download Scientific Diagram
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/QXVmVDZfQ29GV1k=/sd/)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
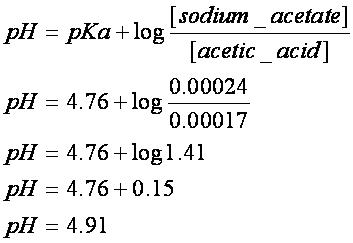
Calculate pH of 100 ml buffer solution containing 0.1 g acetic acid and 0.2 g sodium actetate. | pH calculations and more in fundamentals of pharmaceutics.

A 0.01 M solution of acetic acid is 1.34 % ionized (degree of dissociation = 0.0134 ) at 298 . What is the ionization constant of acetic acid.
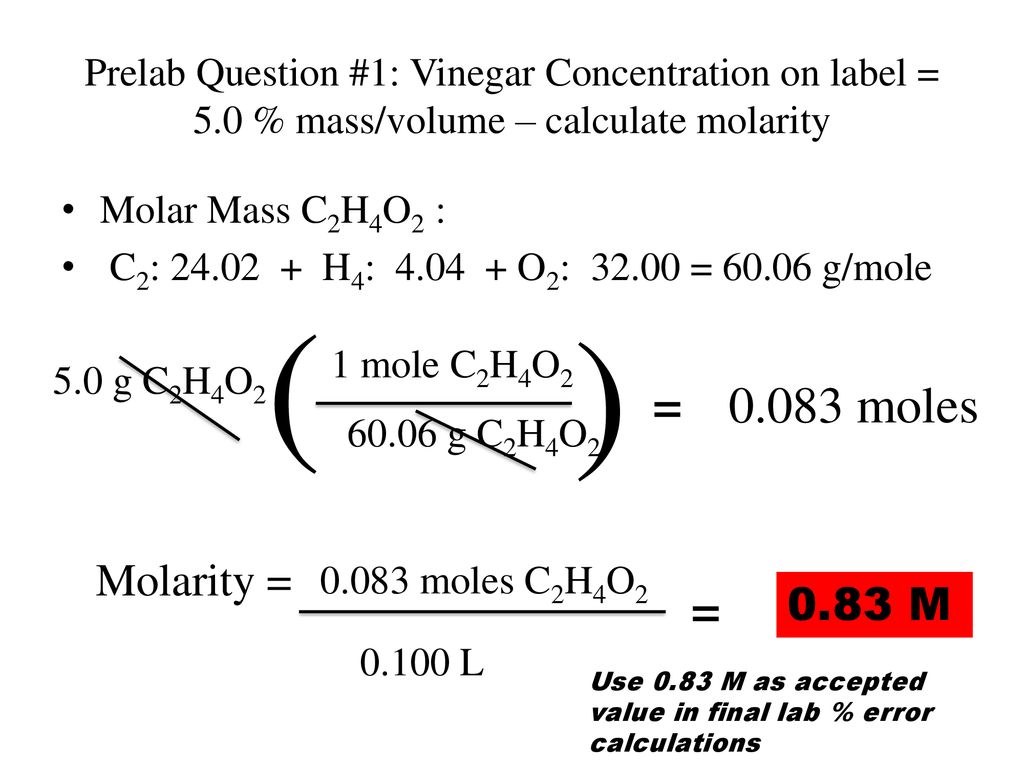
Titration What is the concentration in moles/liter of a vinegar (solution of acetic acid in water)? - ppt download
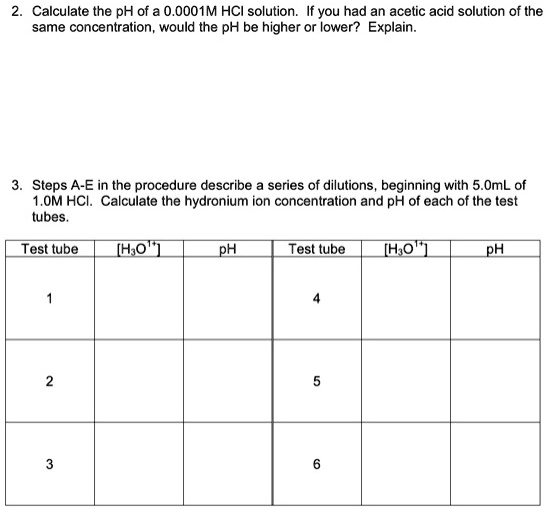
SOLVED: Calculate the pH of 0.0OO1M HCI solution If you had an acetic acid solution of the same concentration, would the pH be higher Or lower? Explain. Steps A-E in the procedure
What is the pH of 0.1 m of acetic solution, if acetic acid is a weak acid with Ka2 1.86 × 10-5? - Quora
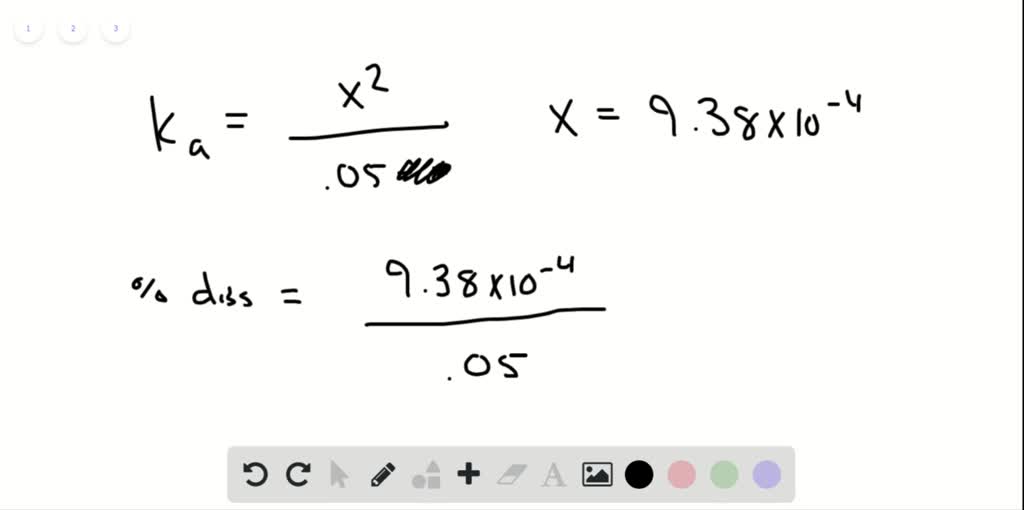
SOLVED:Calculate the percent dissociation of the acid in each of the following solutions. a. 0.50M acetic acid b. 0.050M acetic acid c. 0.0050M acetic acid d. Use Le Châtelier's principle to explain
















