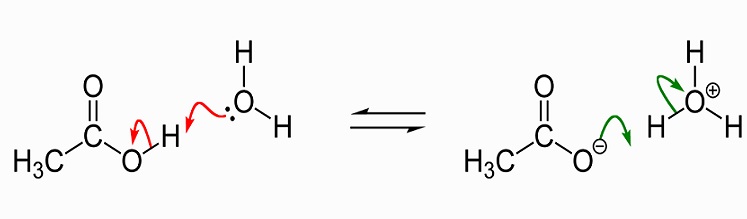
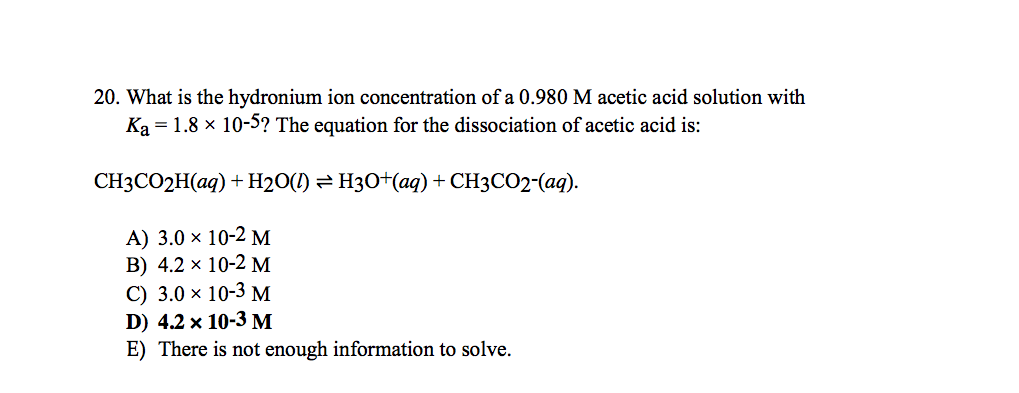
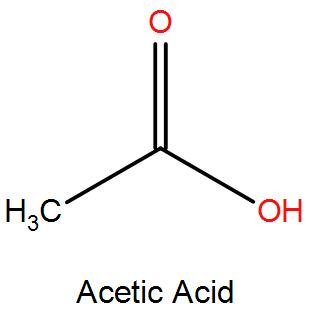
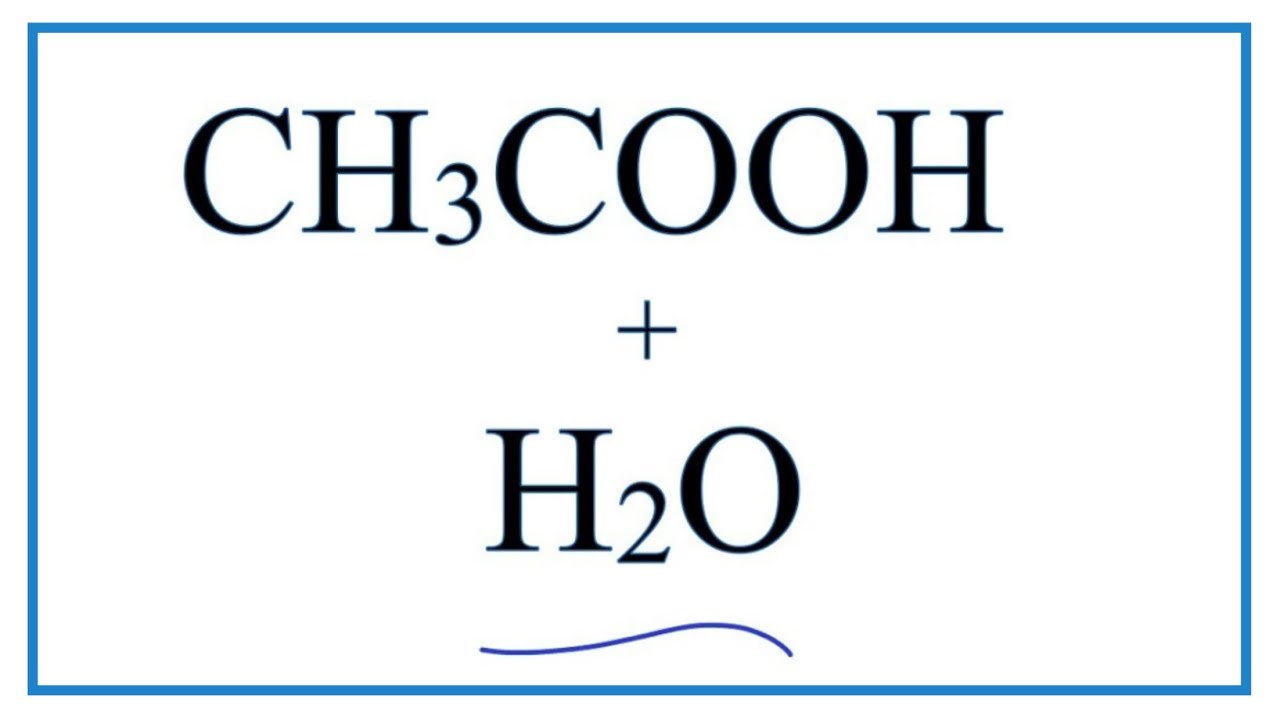
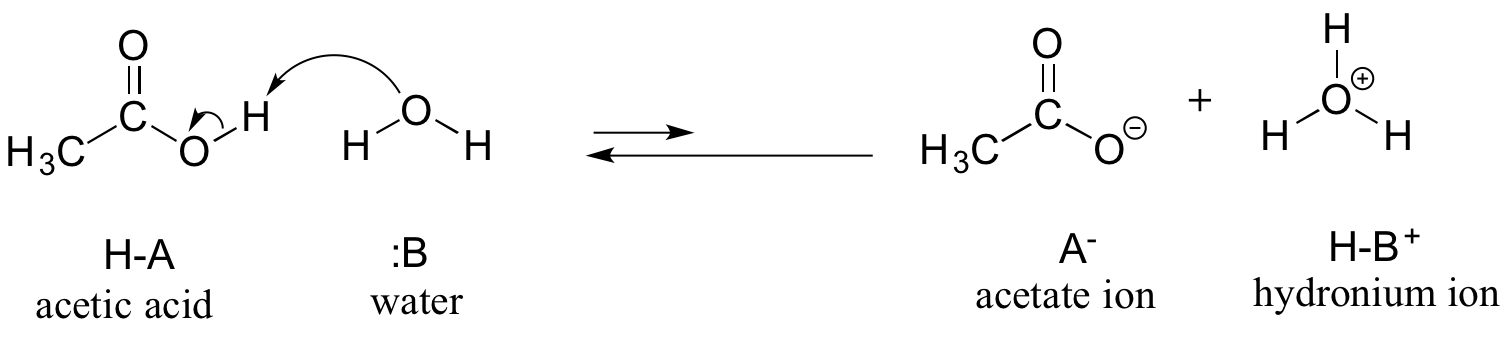
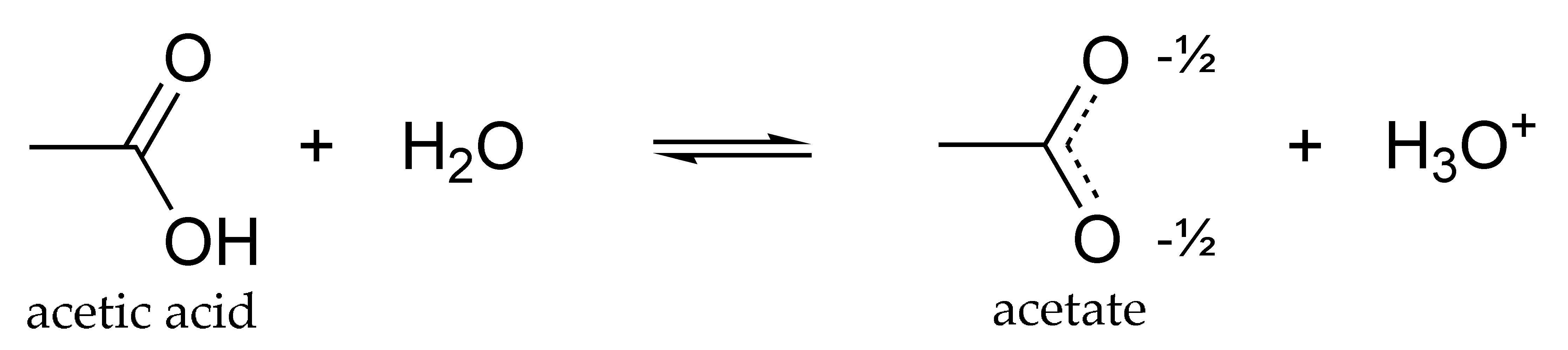SOLVED: Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aquesous solution: HCzH3Ozlaq) = CzH3Oz (aq) + Ht(aq) At equilibrium at 259C a 0.102M solution

Chart 1). Elimination of acetic acid from the molecular ions led to the... | Download Scientific Diagram

SOLVED: What Is the complete ionic, net ionic equation and the spectator ion for the acid- base reaction that occurs when acetic acid and potassium hydroxide solutions are mixed? (4 Points) (d)CH;COO- (

In acetic acid if acetate ion is resonance stabilized how can the electrons pushed by methyl group destabilize the anion? - Find 8 Answers & Solutions | LearnPick Resources
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora