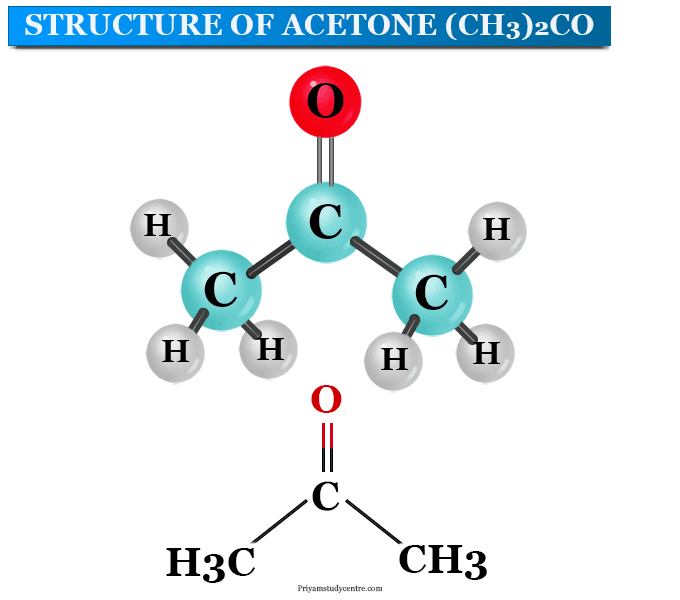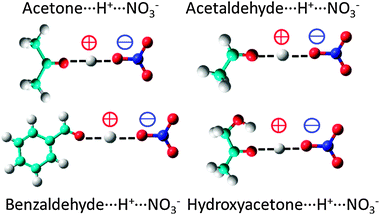
Proton transfer reactions between nitric acid and acetone, hydroxyacetone, acetaldehyde and benzaldehyde in the solid phase - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C2CP42033C

PDF) Proton transfer reactions between nitric acid and acetone, hydroxyacetone, acetaldehyde and benzaldehyde in the solid phase

Dr Luke Gamon on Twitter: "Pure copper, concentrated nitric acid & dry ice / acetone cold bath. About to get crazy up in here! #RealTimeChem http://t.co/pF82sk0AUt" / Twitter