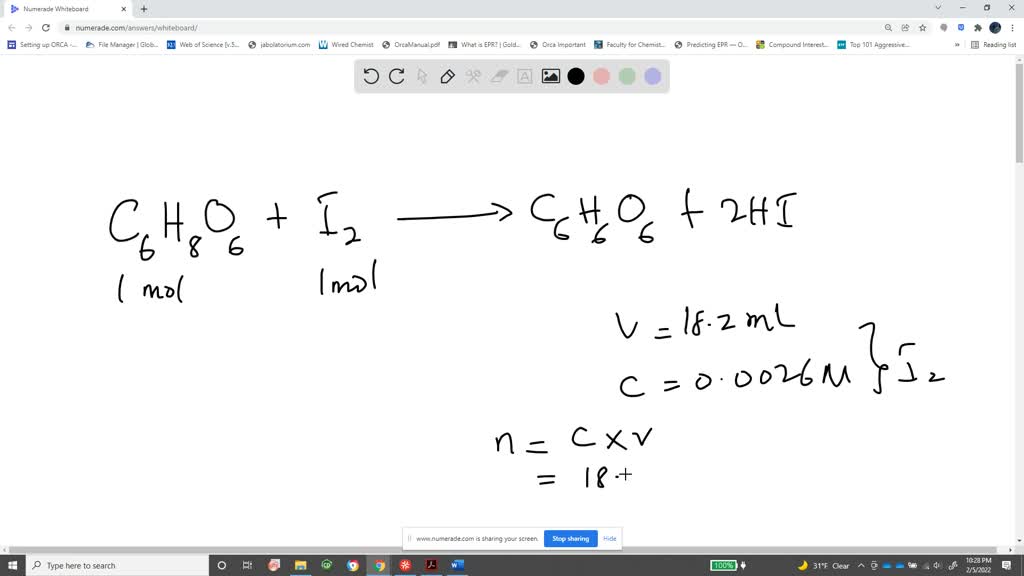
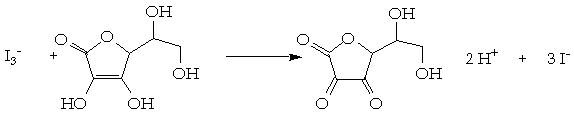
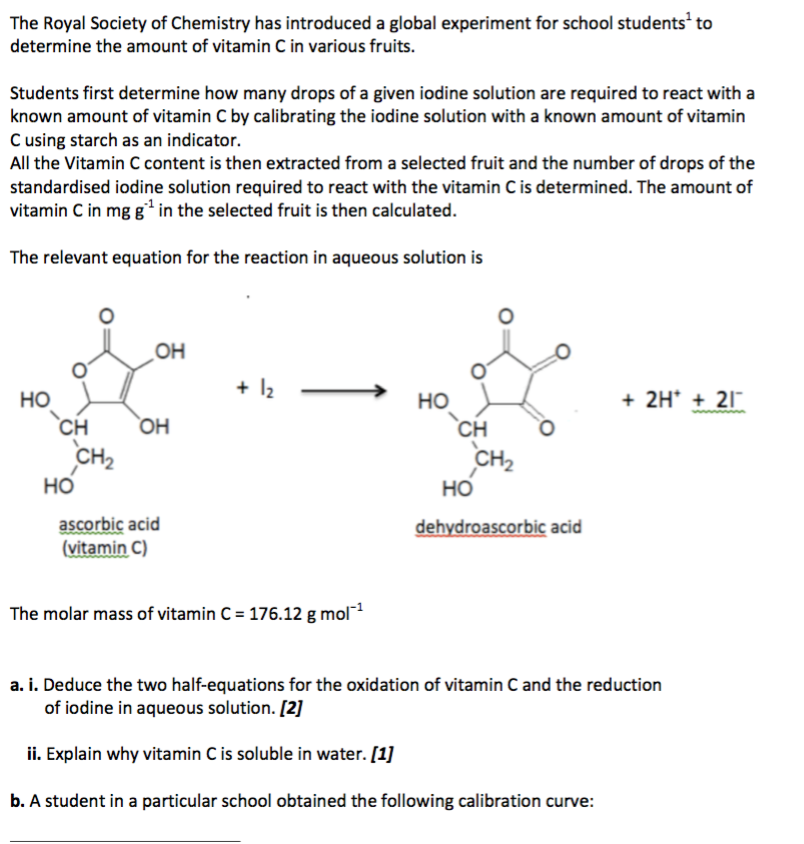
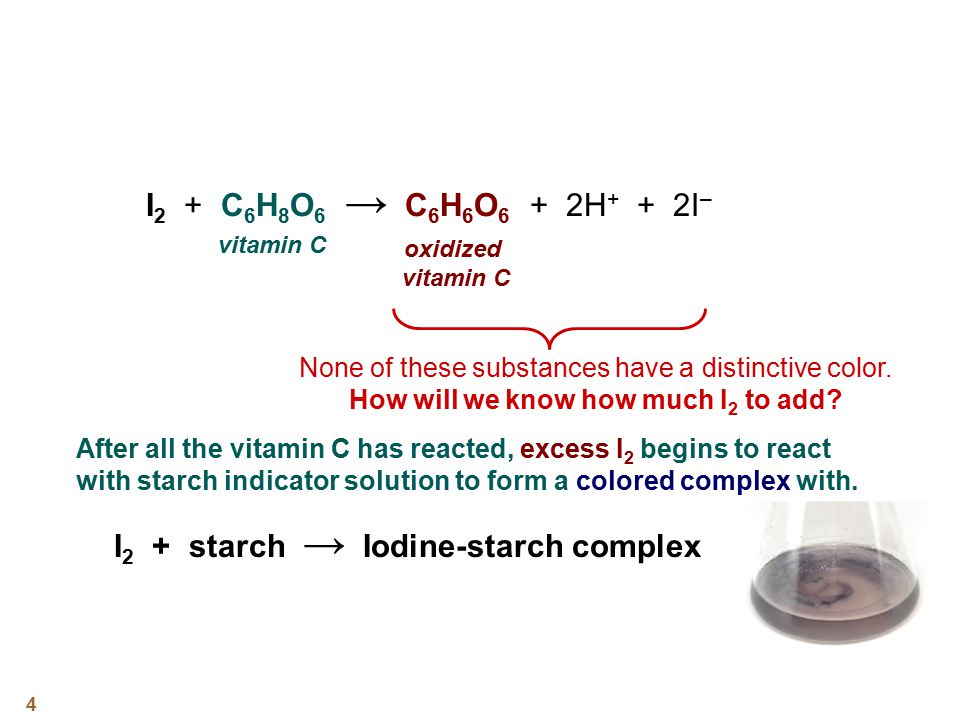
SOLVED: How to calculate vitamin C from a redox titration? Reagents: 1mL 1% starch indicator 2.5 mL of HCl 5mL 10% potassium iodide Potassium iodate 14.05mL (0.006M) (I did 4 titrations and
I'm titrating 2 types of orange juices to see how the vitamin C content differs. How would I know how many drops of starch and ml of juice I have to use

Using a classical method of vitamin C quantification as a tool for discussion of its role in the body - ScienceDirect
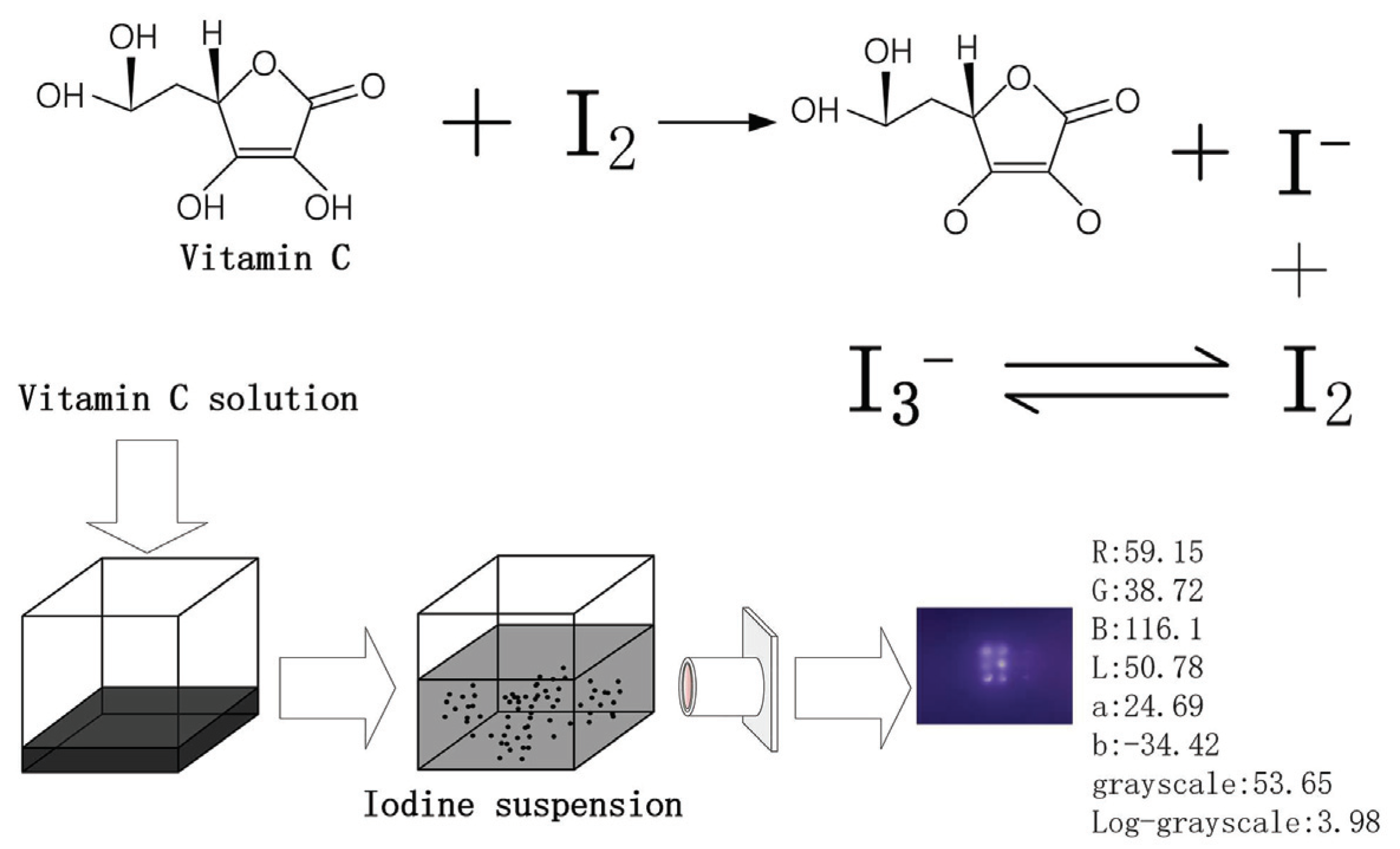
Applied Sciences | Free Full-Text | Determination of Vitamin C in Foods Using the Iodine-Turbidimetric Method Combined with an Infrared Camera

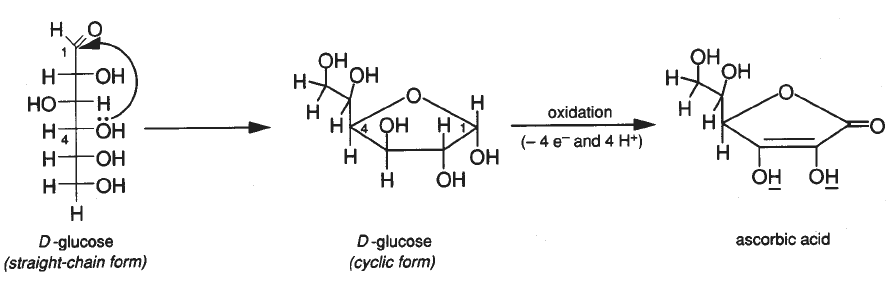
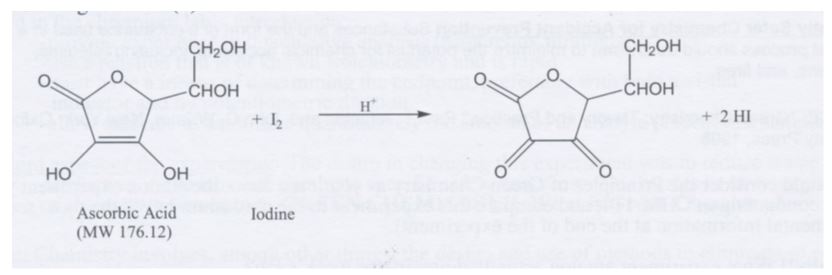
Structures for ascorbic acid and for the reduced form (dihydroascorbic... | Download Scientific Diagram