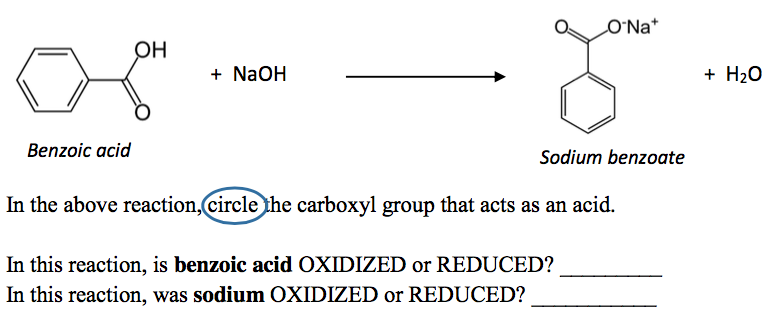Titration curves of benzoic acid for different analyte concentrations... | Download Scientific Diagram
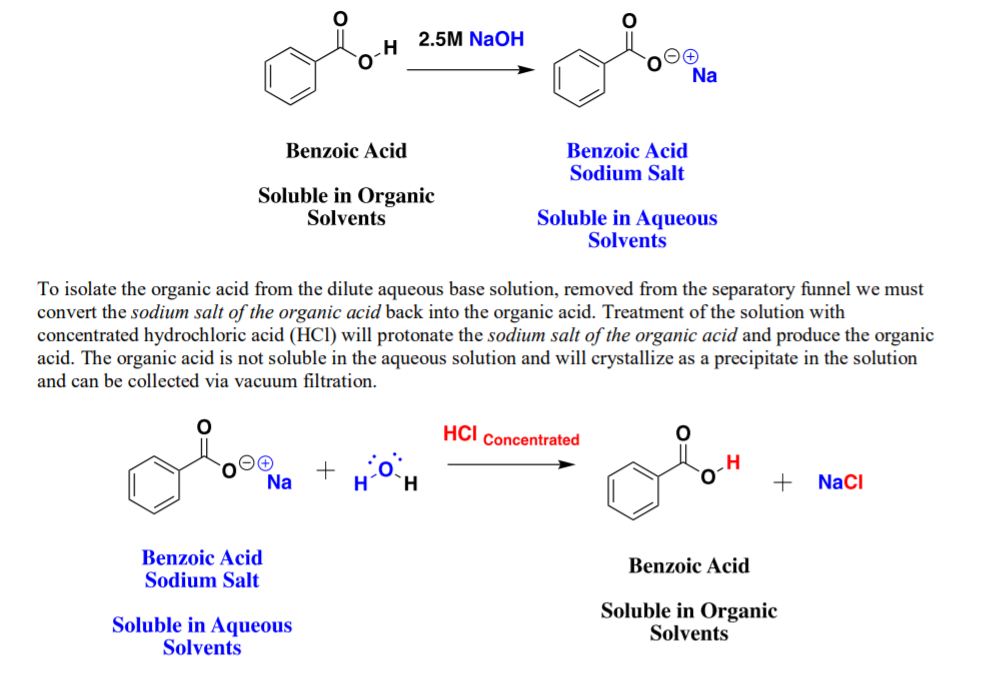
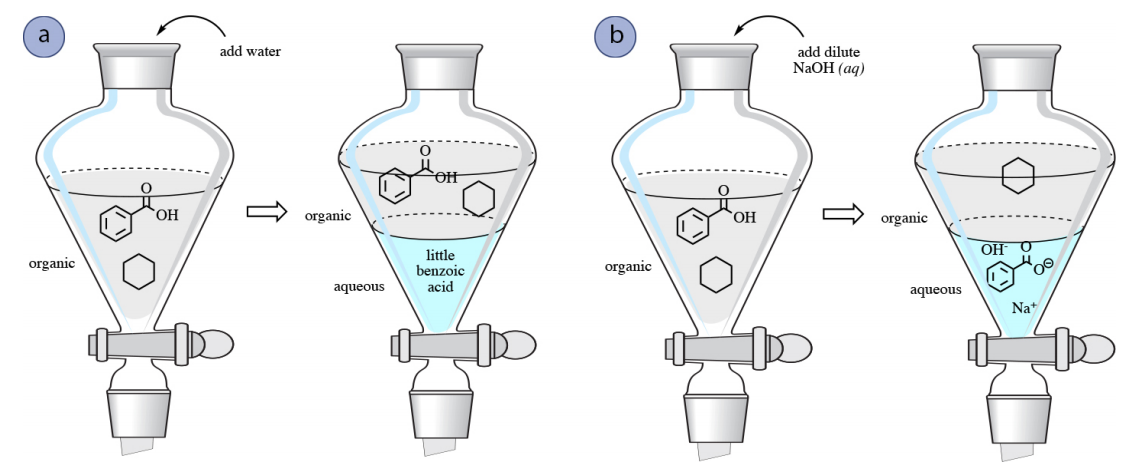
✓ Solved: When benzoic acid ( 5 ) is partitioned between diethyl ether and aqueous sodium hydroxide solution...

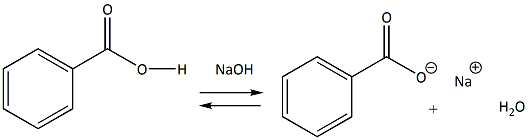
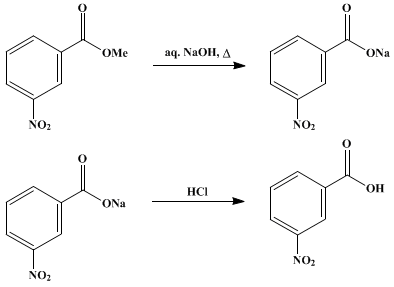
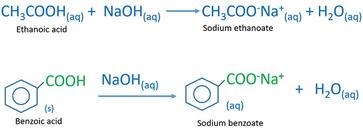
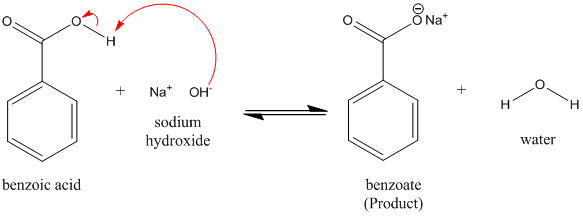
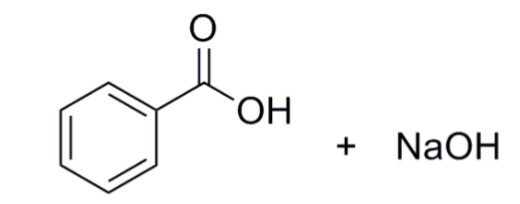
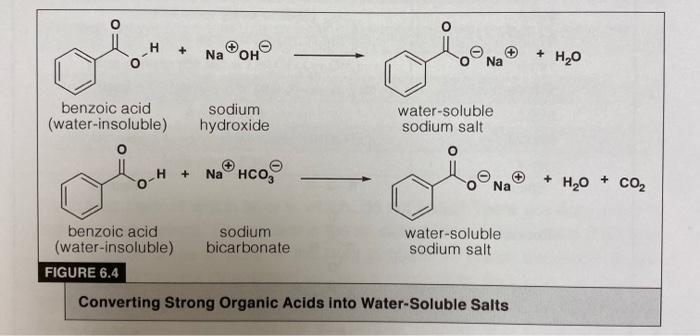
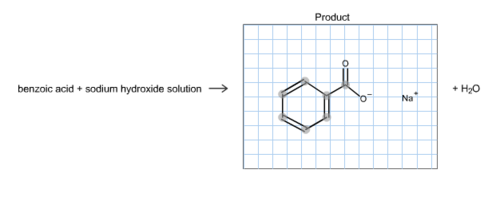
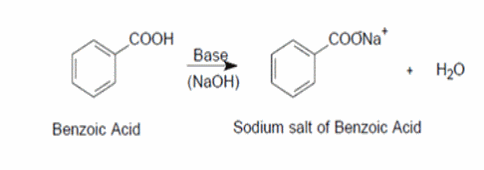
Draw a balanced reaction equation (using structures) for the reaction between benzoic acid and aqueous sodium hydroxide. | Homework.Study.com
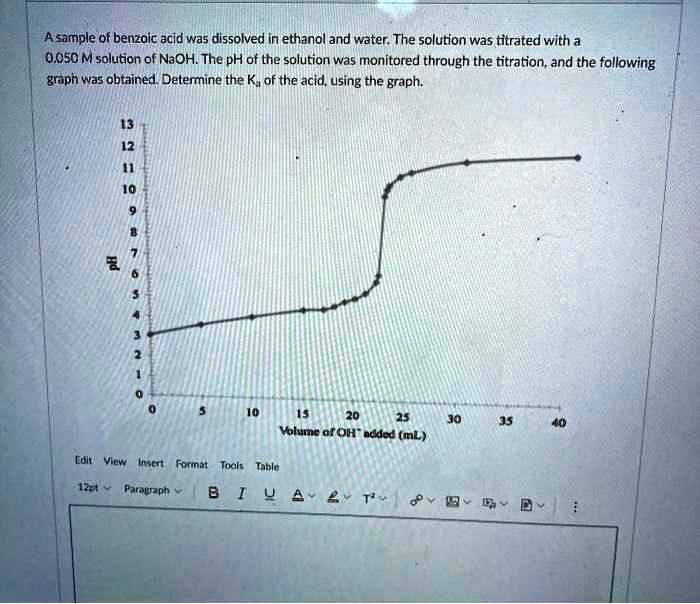
SOLVED: A sample of benzoic acid was dissolved in ethanol and water: The solution was titrated with a 0.O5O M solution of NaOH The pH of the solution was monitored through the
Choose the correct representation of conductometric titration of benzoic acid vs sodium hydroxide. - Sarthaks eConnect | Largest Online Education Community

SOLVED: A solution of benzoic acid (HC,HsOz) is titrated using sodium hydroxide: The Ka of benzoic acid is 6.4x 101. Write the balanced equation and the net ionic equation. 2.100.0 mL of

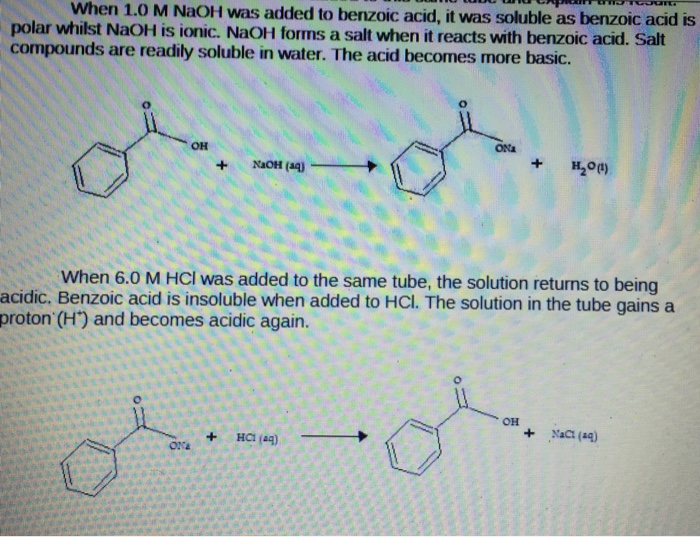
Explain the results for the tube in which 1.0 m naoh was added to benzoic acid. write an equation for this, - Brainly.com
![When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] . When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .](https://haygot.s3.amazonaws.com/questions/1835171_829774_ans_40c76abc129644cfaff5cbb818338569.png)
When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .