
Facile Hydrothermal Fabrication of an α-Ni(OH)2/N-Doped Reduced Graphene Oxide Nanohybrid as a High-Performance Anode Material for Lithium-Ion Batteries | Energy & Fuels
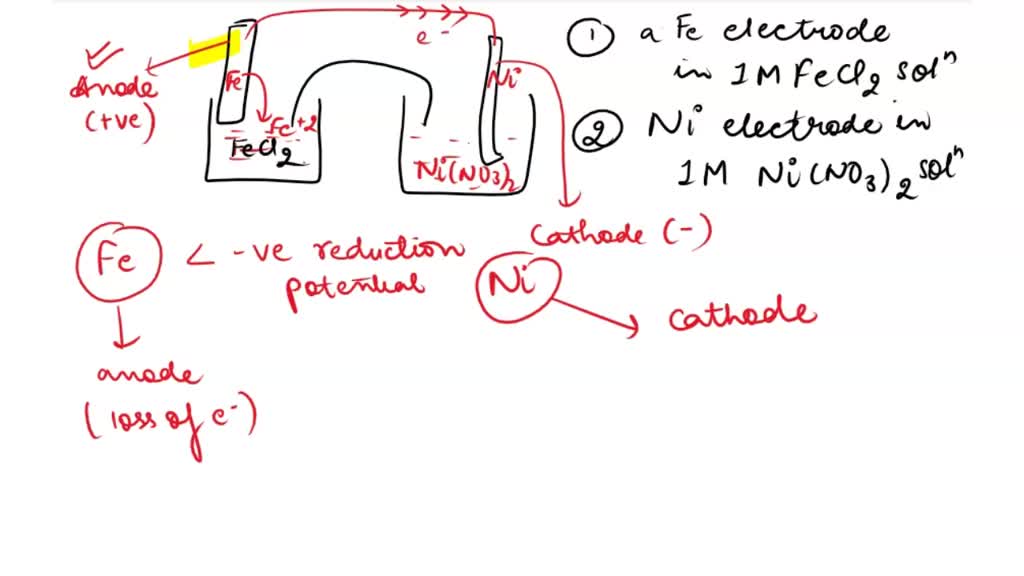
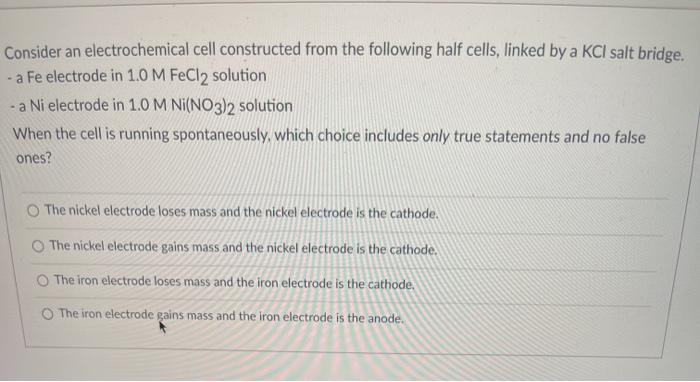
SOLVED: Consider an electrochemical cell constructed from the following half cells, linked by a KCl salt bridge. • a Fe electrode in 1.0 M FeCl2 solution • a Ni electrode in 1.0

SOLVED: These compounds are in water: State the compound that forms a precipitate or indicate 'no precipitate' NaCl + Ba(OH)2 AgCIO3 Mgl2 CaCl2 K2CO3 FeCl2 (NH4)2S04 Ni(NO3)2 KOH Ni(NO3)2 K25 Pb(NO3)2 K2CO3
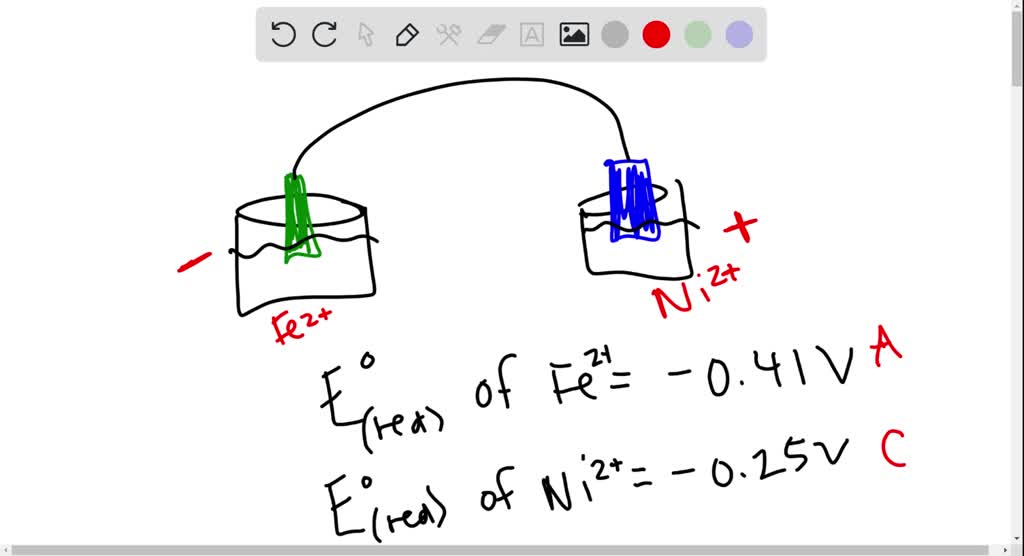
SOLVED: Consider an electrochemical cell constructed from the following half cells, linked by a KCl salt bridge: -a Fe electrode in 1.0 M FeCl2 solution -a Ni electrode in 1.0 M Ni(NO3)2
![PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a2e575c3a179f867fc9dc267d3158d61b9044f86/2-Figure1-1.png)
PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar
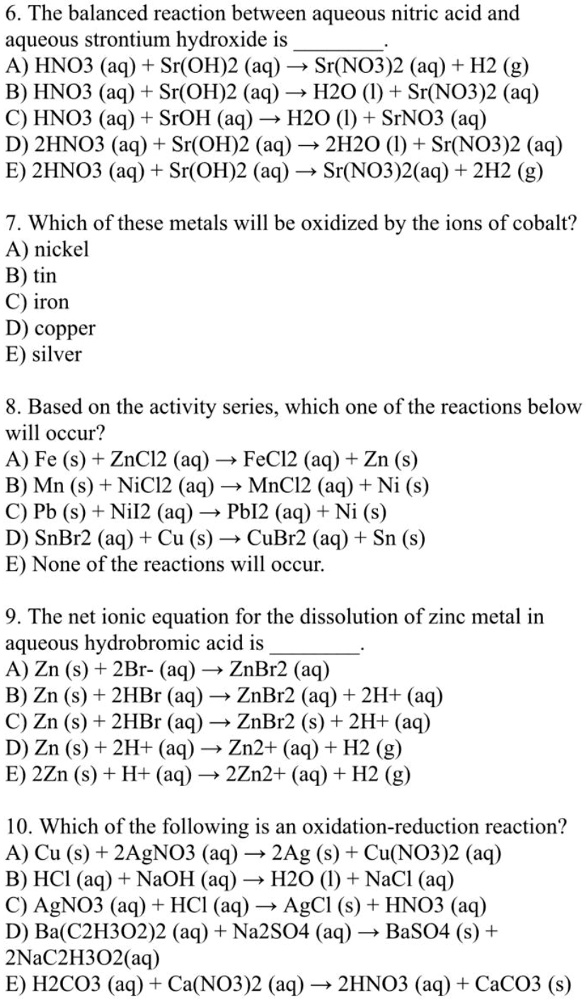
SOLVED: The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is HNO3 (aq) + Sr(OH)2 (aq) 57 Sr(NO3)2 (aq) + H2 (g) B) HNO3 (aq) + Sr(OH)2 (aq) 5 H2O (

An electrochemical cell is constructed using 1.0 M solutions of Fe(NO3)2 and Sn(NO3)2 and strips of Fe and Sn. A voltmeter is connected to the electrodes and a salt bridge connects the
![PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar PDF] Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a2e575c3a179f867fc9dc267d3158d61b9044f86/2-Table1-1.png)












![Synthesis of novel thiazolo[3,2‐a]chromeno[4,3‐d]pyrimidine‐6(7H)‐on Synthesis of novel thiazolo[3,2‐a]chromeno[4,3‐d]pyrimidine‐6(7H)‐on](https://www.researcher-app.com/image/eyJ1cmkiOiJodHRwczovL3MzLWV1LXdlc3QtMS5hbWF6b25hd3MuY29tL3N0YWNrYWRlbWljL3Byb2R1Y3Rpb24vcGFwZXIvNDAzMTI2Mi5wbmciLCJmb3JtYXQiOiJ3ZWJwIiwicXVhbGl0eSI6MTAwLCJub0NhY2hlIjp0cnVlfQ==.webp)