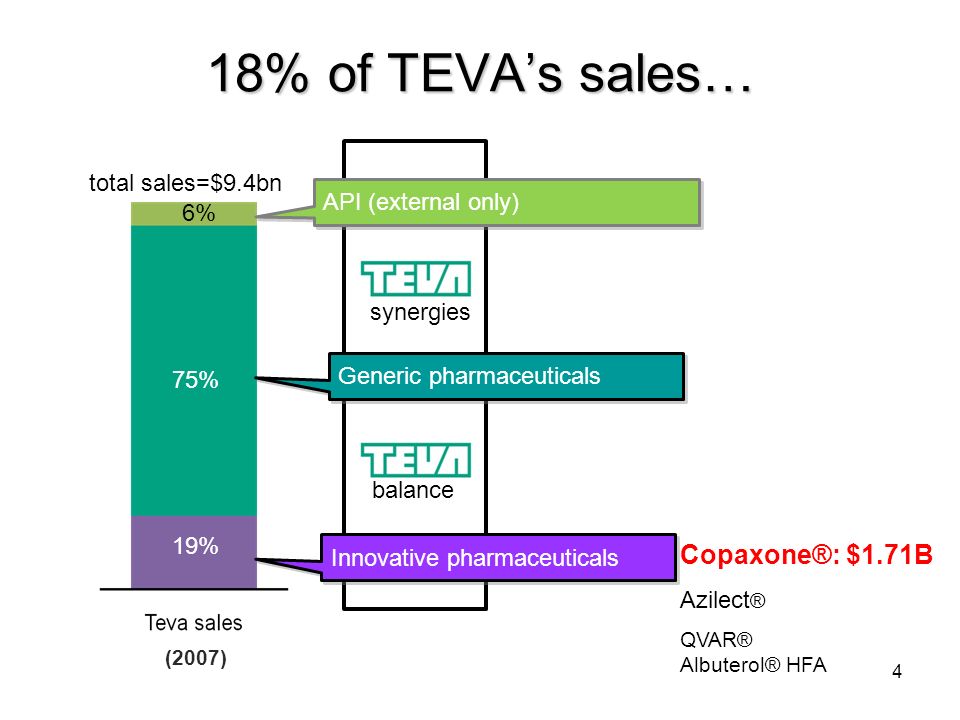
Teva Pharmaceuticals v. Sandoz: availability of generic glatiramer acetate and the impact to patent litigation claim constructio

Mylan Invalidates Two of Teva's Copaxone® 40 mg/mL Patents Via U.S. Patent and Trademark Office's Inter Partes Review Proceeding
February 25, 2008 Decision: PMPRB-06-D2-COPAXONE IN THE MATTER OF the Patent Act, R.S.C. 1985, c. P-4, as amended AND IN THE MA