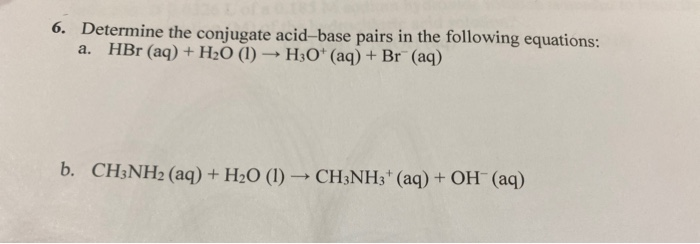Acid-Base Reactions. Definitions Strong Acids HCl HBr HI HNO 3 HClO 4 H 2 SO 4 Acid: a species that supplies H + ions to water Strong acid: - ppt download
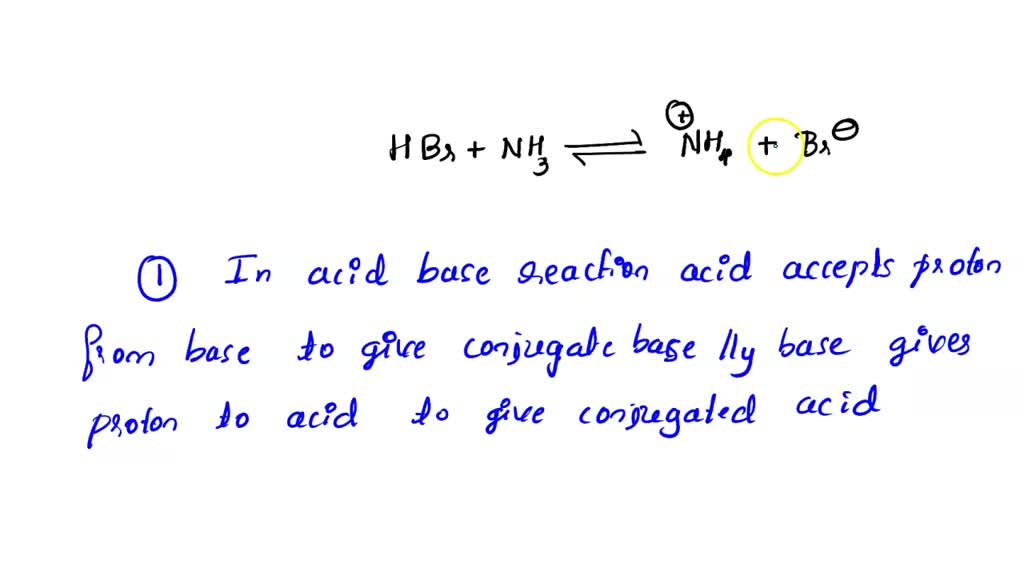
SOLVED: Draw the curved arrows and the products formed in the acid base reaction of HBr and NHz: Determine the direction of equilibrium Step 1: What happens in an acid-base reaction? Step
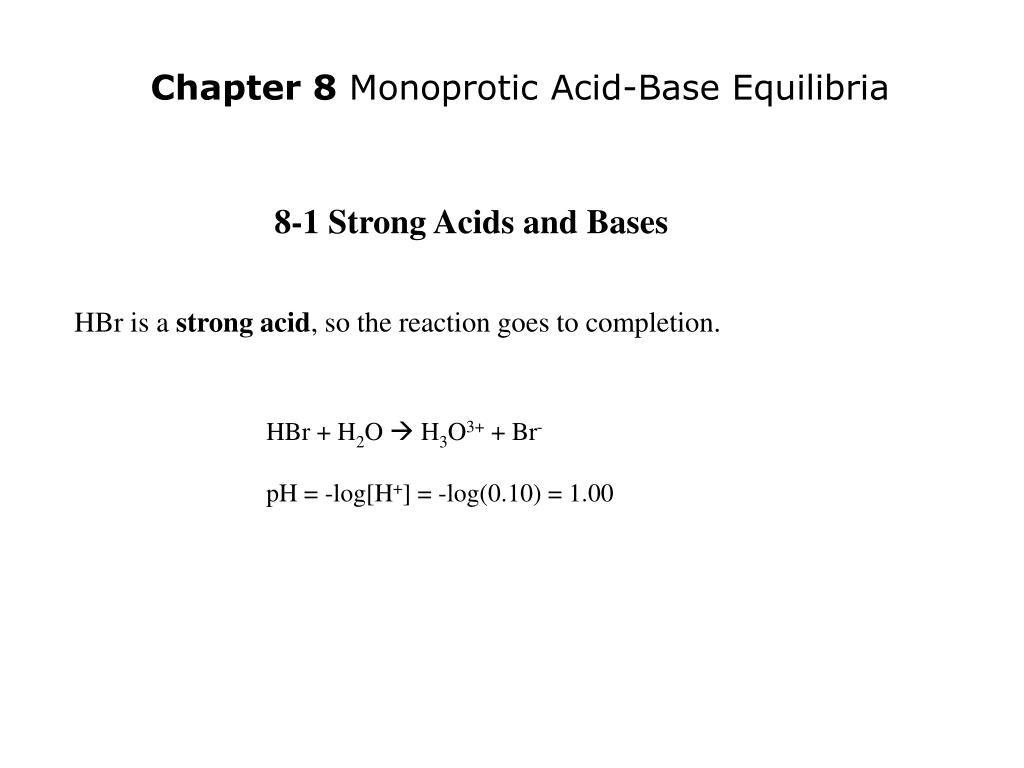
PPT - HBr is a strong acid , so the reaction goes to completion. PowerPoint Presentation - ID:4536389
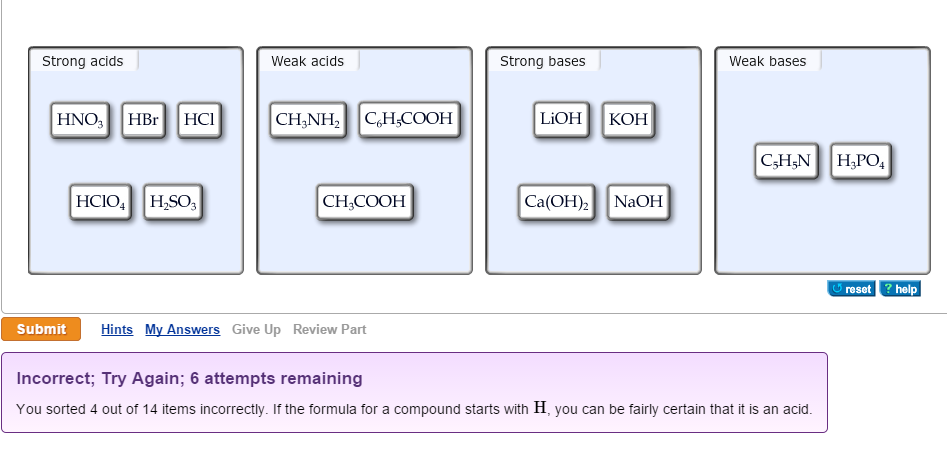
CHM 201 ACID/BASE PRACTICE The following are Bronsted-Lowry acid/base reactions. Identify the acid, the base and draw the conju

PHILIPPINE CHEMISTRY PROFESSIONALS BOARD EXAM REVIEWER - Which one of the following mechanistically depicts the acid-base reaction that occurs when hydrobromic acid(HBr) is added to methanol (CH4O)? Please refer to attached image.
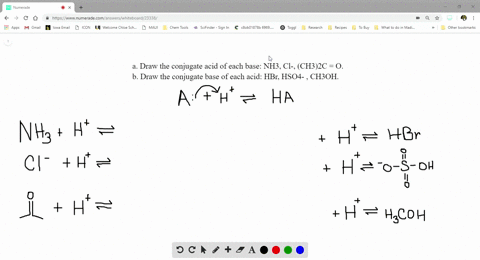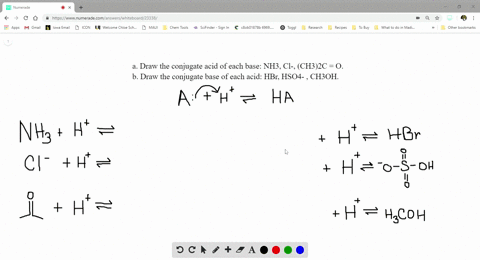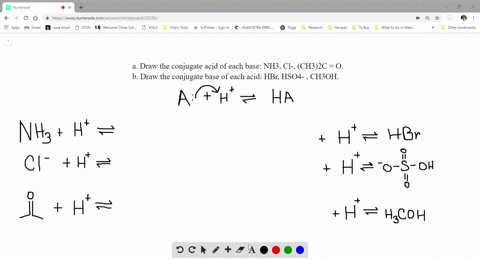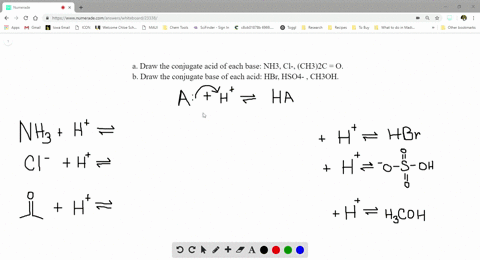
SOLVED:a. Draw the conjugate acid of each base: NH3, Cl^-, (CH3)2C = O. b. Draw the conjugate base of each acid: HBr, HSO4^- , CH3OH.
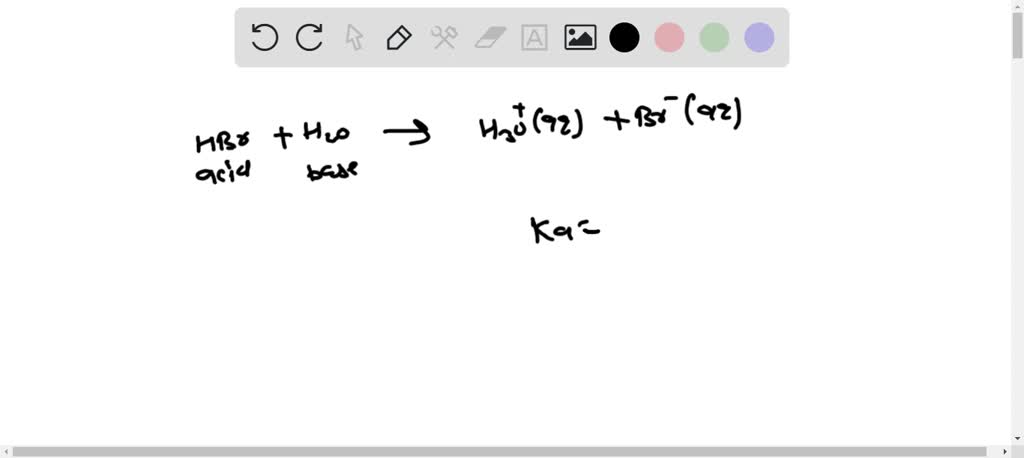
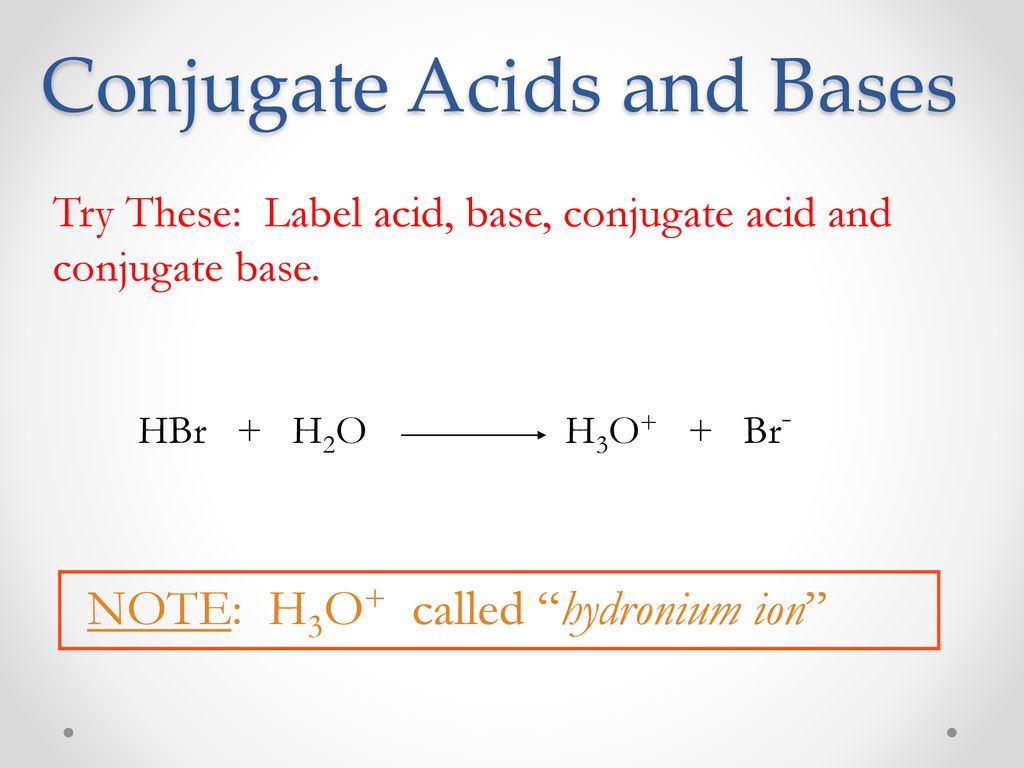
SOLVED: (a) write the net acid-base reaction that occurs when HBr is added to water. (use the lowest possible coefficients. Omit states-of-matter in your answer.)(b) What is the relative Ka value for

PPT - HCl (aq) hydrochloric acid HBr (aq) hydrobromic acid HI (aq) hydroiodic acid PowerPoint Presentation - ID:3967608
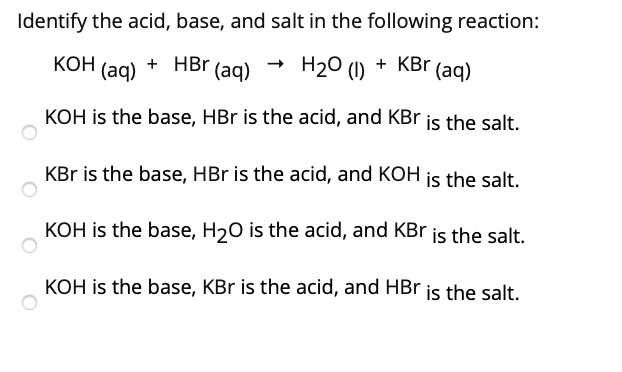
SOLVED: Identify the acid, base, and salt in the following reaction: KOH (aq) HBr (aq) HzO () KBr (aq) KOH is the base, HBr is the acid, and KBr is the salt