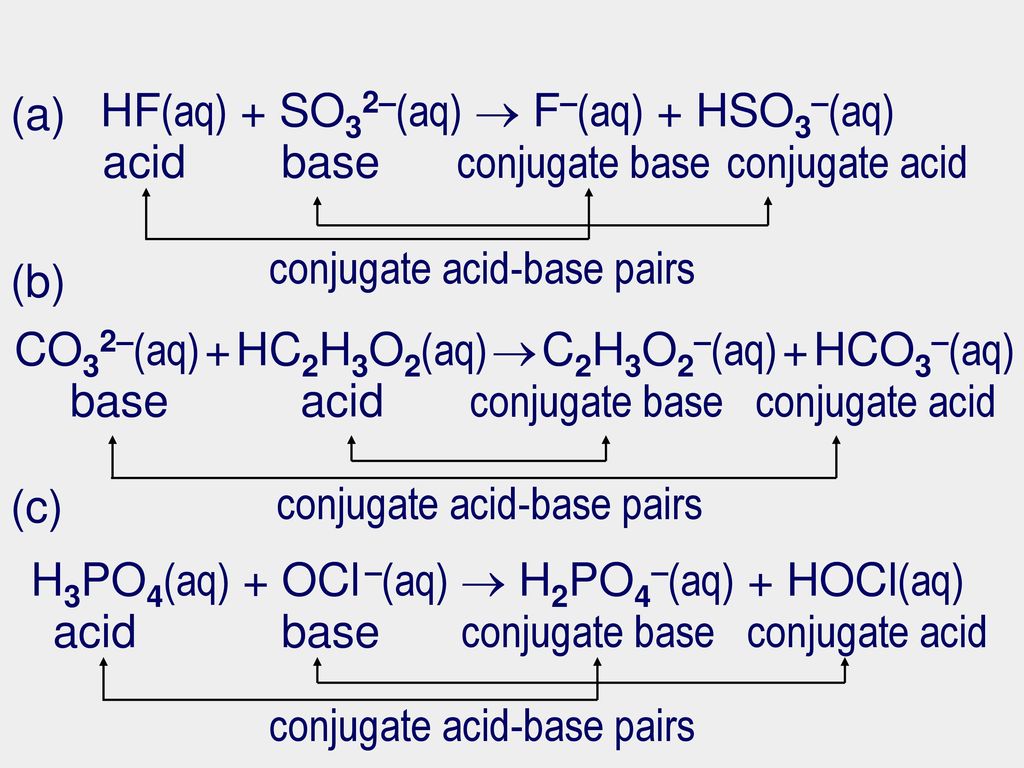
Practice problems Identify the acid, base, conjugate acid, conjugate base, and conjugate acid-base pairs: HC2H3O2(aq) + H2O(l) C2H3O2–(aq) + H3O+(aq) - ppt video online download
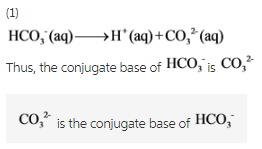
What is the conjugate base of HCO3−? Express your answer as a chemical formula - Home Work Help - Learn CBSE Forum

What is the conjugate base of HCO3−? Express your answer as a chemical formula - Home Work Help - Learn CBSE Forum
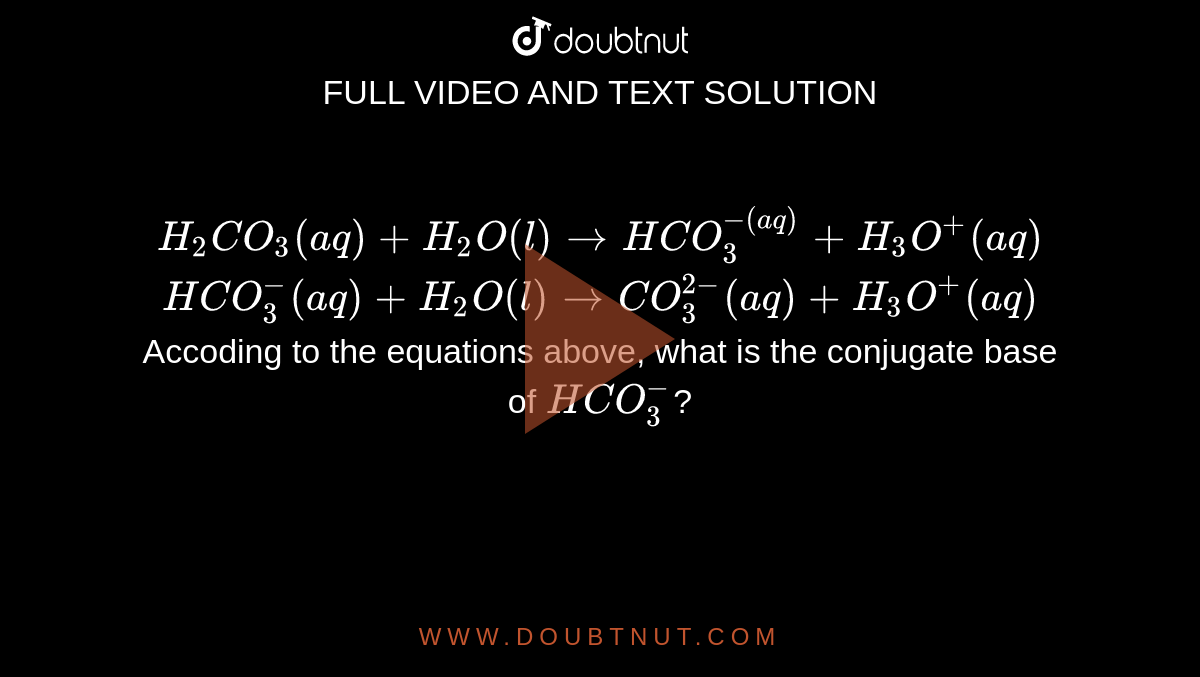
H2CO3(aq)+H2O(l)toHCO3^-(aq) + H3O^+(aq) HCO3^(-)(aq) + H2O(l)to CO3^(2-)(aq)+H3O^(+)(aq) Accoding to the equations above, what is the conjugate base of HCO3^-?

The species: H2O, HCO3^-, HSO4^- and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.

Identify the conjugate acid-base pairs in the following reaction. Indicate what each substance is in each pair. H2CO3 + PO43- arrow HCO3- + HPO42- | Homework.Study.com

SOLVED: The hydrogen carbonate ion, HCO3 , has both a conjugate acid and a conjugate base. These are; respectively: CO;? HCO; Hzot, OH HzCO;, CO; HCO;, HCO; HCO; HCO;





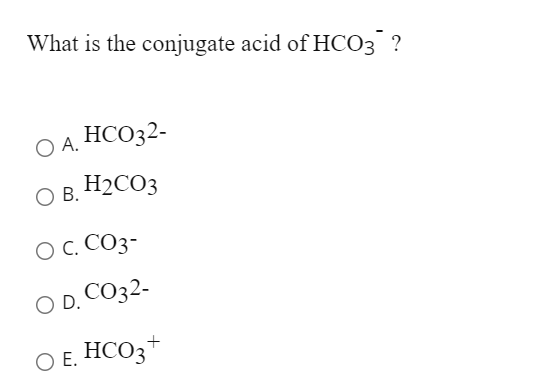
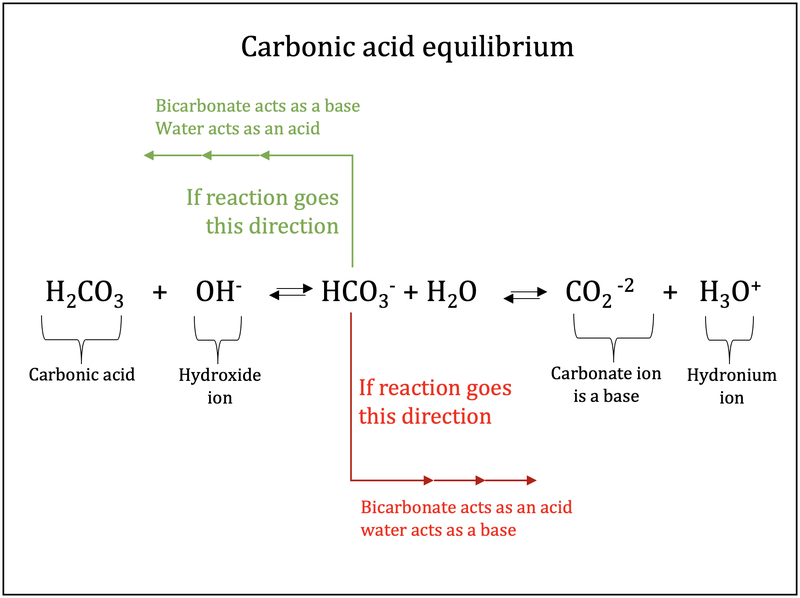

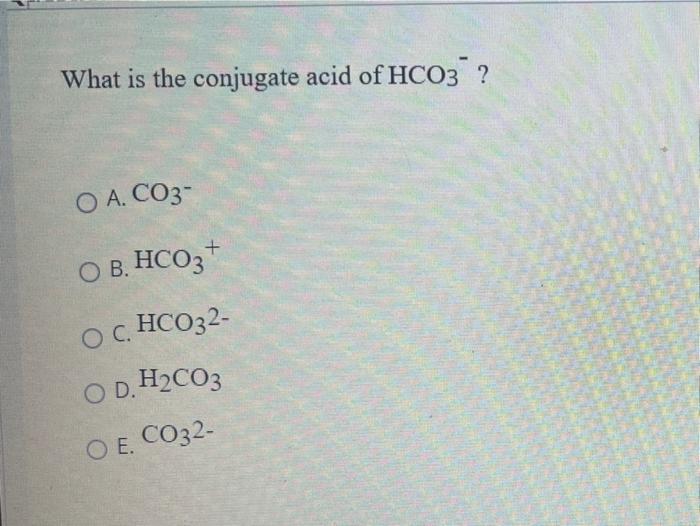


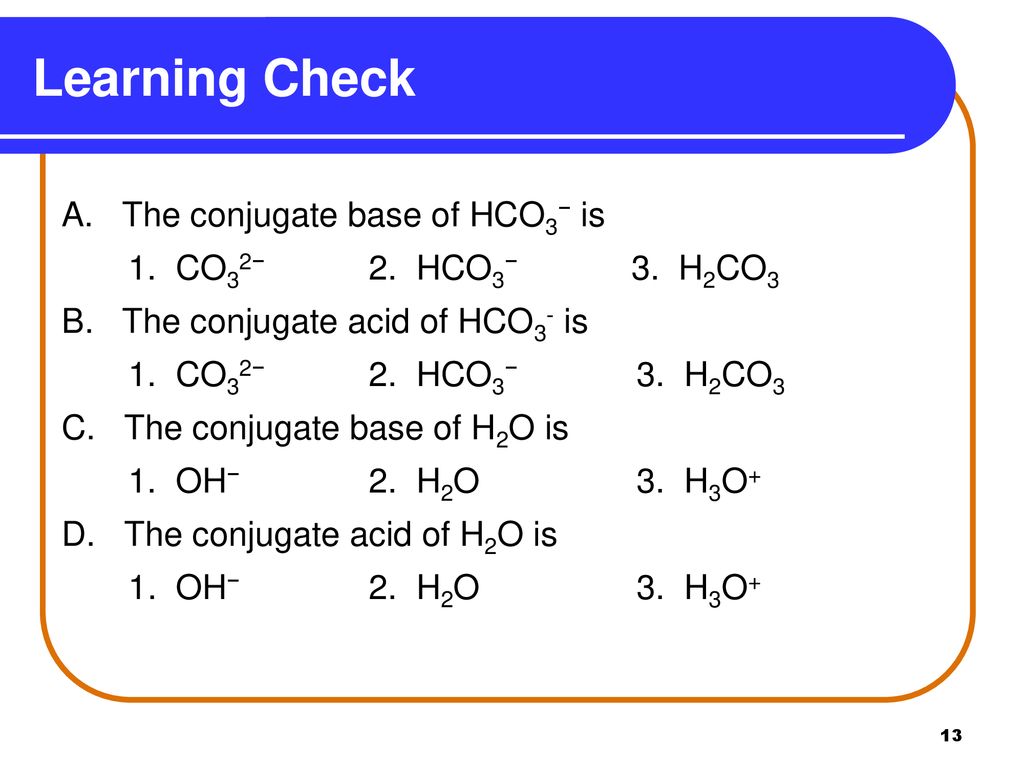
![ANSWERED] HCO3 + H₂→ H₂CO3 + OH In the equation abov... - Organic Chemistry ANSWERED] HCO3 + H₂→ H₂CO3 + OH In the equation abov... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/44878390-1658615733.1594505.jpeg)
