Molar Mass = mass in grams of one mole –Units grams/mole For elements, molar mass = atomic mass Why aren't all atomic masses whole numbers? –Remember that. - ppt download
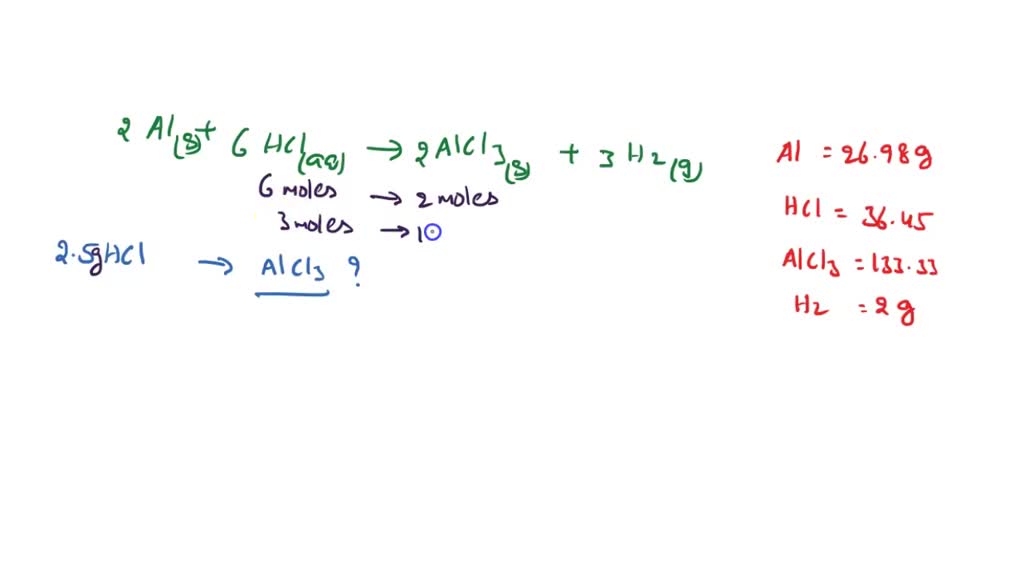
SOLVED: 2Al(s)+ 6HCl(aq)—-> 2AlCl3(s) + 3H2(g) Molar mass Al=26.98 g/mol Molar mass HCl= 36.45 Molar mass AlCl3= 133.33 g/mol Molar mass H2= 2.00g/mol If 2.5 grams of hydrochloric acid completely react to
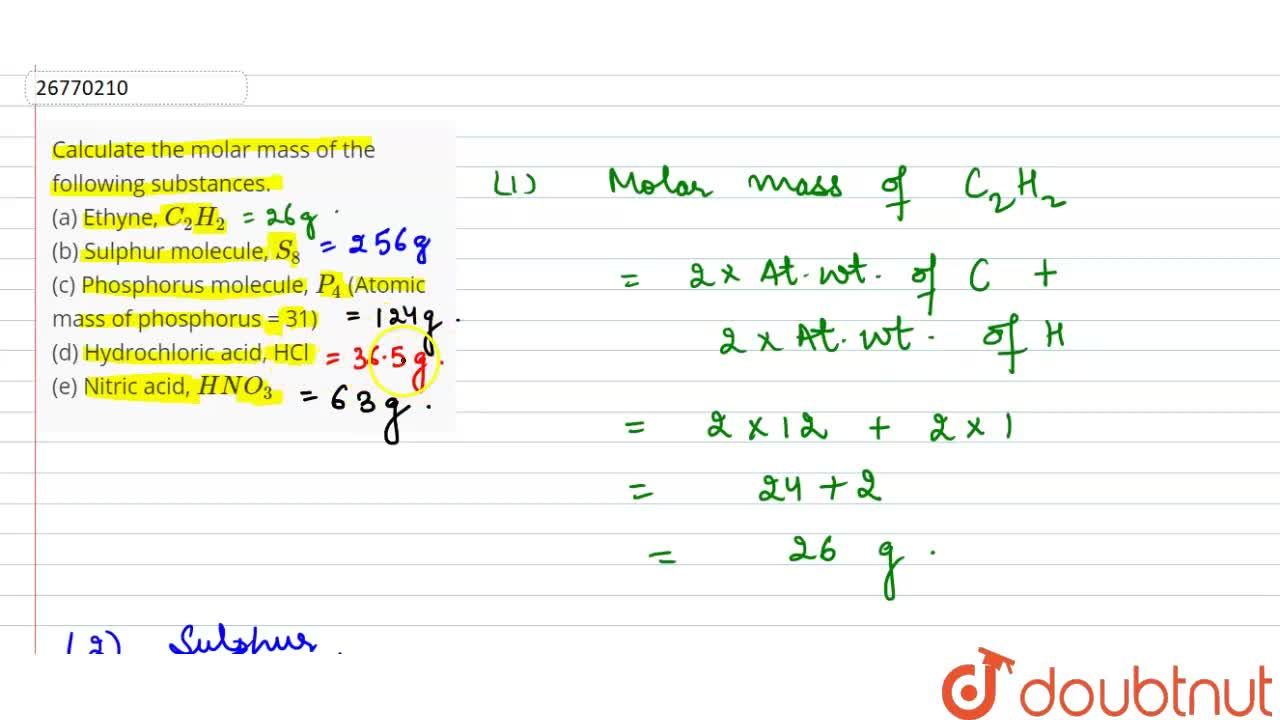
Calculate the molar mass of the following substances. (a) Ethyne, C(2)H(2) (b) Sulphur molecule, S(8) (c) Phosphorus molecule, P(4) (Atomic mass of phosphorus = 31) (d) Hydrochloric acid, HCl (e) Nitric acid,

Fisher Science Education Hydrochloric Acid, 0.1M (0.1N) Solution, Quantity: 500mL | Fisher Scientific

HCl Acid (Hydrochloric acid) - Structure, Molecular mass, Preparations, Properties and Videos with FAQs of Hydrochloric acid.