Continuous production of magnetic iron oxide nanocrystals by oxidative precipitation - ScienceDirect

Arsenate co-precipitation with Fe(II) oxidation products and retention or release during precipitate aging - ScienceDirect

Synthesis, structural, morphological, optical and magnetic characterization of iron oxide (α-Fe2O3) nanoparticles by precipitation method: Effect of varying the nature of precursor - ScienceDirect

Iron Oxide Nanoparticles and Nano-Composites: An Efficient Tool for Cancer Theranostics | IntechOpen
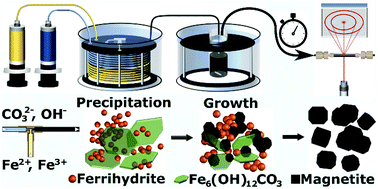
Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-Ray diffraction in solution - Nanoscale (RSC Publishing)

Uranium(V) Incorporation Mechanisms and Stability in Fe(II)/Fe(III) (oxyhydr)Oxides | Environmental Science & Technology Letters

Solubility of Fe 2+ and Fe 3+ species in the pH range of carbonated... | Download Scientific Diagram

Reaction of Iron (II) Sulphate (FeSO4) with Sodium Hydroxide (NaOH) : Precipitation Reaction - YouTube

Physical and magnetic properties of iron oxide nanoparticles with a different molar ratio of ferrous and ferric - ScienceDirect