PDF) Analysis of patient-reported outcomes from the LUME-Lung 1 trial: A randomised, double-blind, placebo-controlled, Phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer
Articles Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer
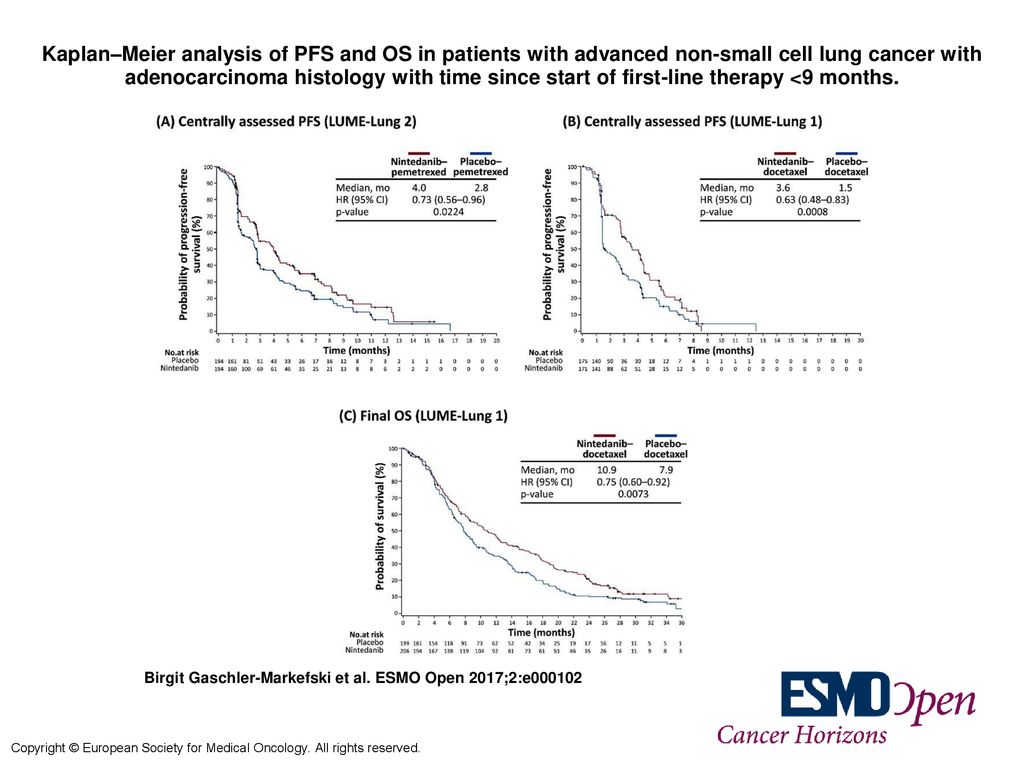
Kaplan–Meier analysis of PFS and OS in patients with advanced non-small cell lung cancer with adenocarcinoma histology with time since start of first-line. - ppt download
Collective teaching of transverse flute as a component of a pulmonary rehabilitation program: An innovative study

Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial - The Lancet Oncology
Time since start of first-line therapy as a predictive clinical marker for nintedanib in patients with previously treated non-sm
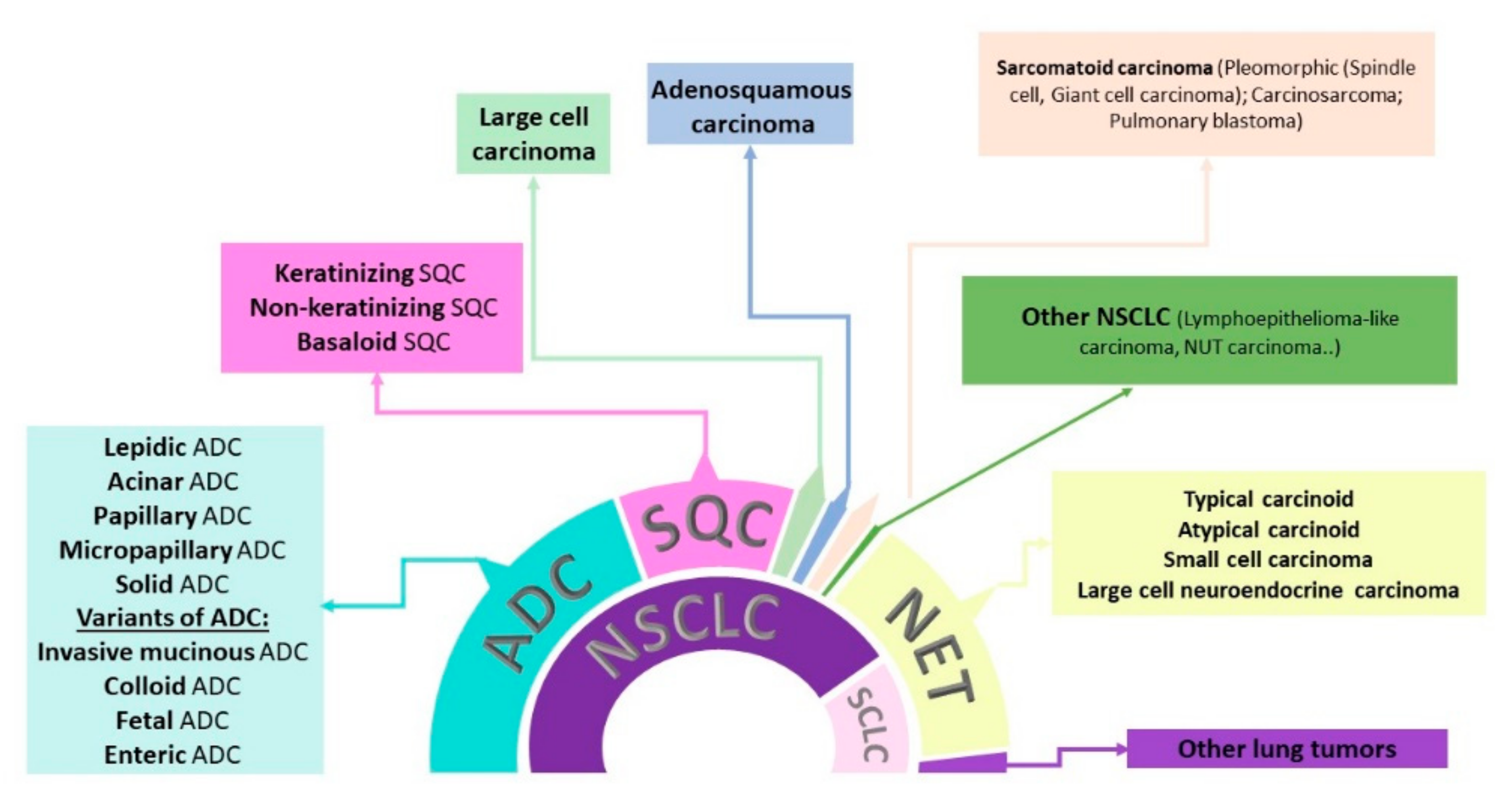
JPM | Free Full-Text | Diagnostic, Predictive, and Prognostic Biomarkers in Non-Small Cell Lung Cancer (NSCLC) Management

Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial - The Lancet Oncology

Analysis of patient-reported outcomes from the LUME-Lung 1 trial: A randomised, double-blind, placebo-controlled, Phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer - ScienceDirect
Investigation of biomarkers in patients with adenocarcinoma of the lung receiving nintedanib according to approved label: Non-in
Boehringer Ingelheim cancer drug shows positive results in Phase III trial - Pharmaceutical Technology
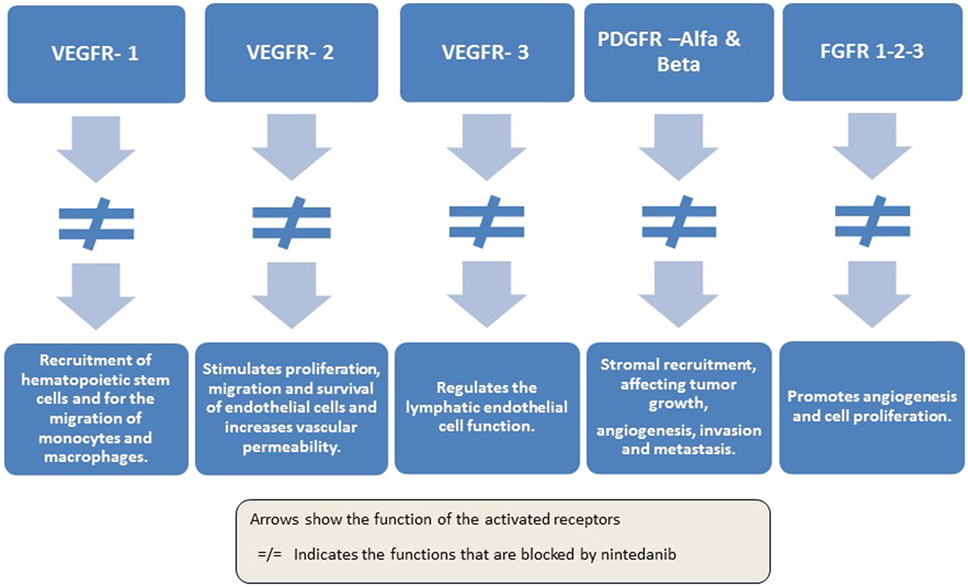
Frontiers | Second-Line Treatment of Non-Small Cell Lung Cancer: Clinical, Pathological, and Molecular Aspects of Nintedanib
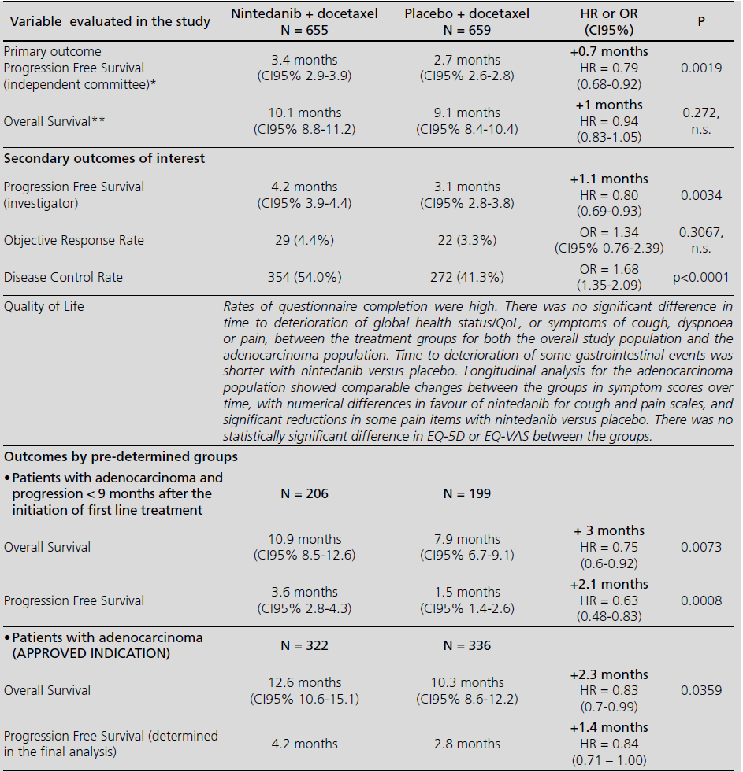
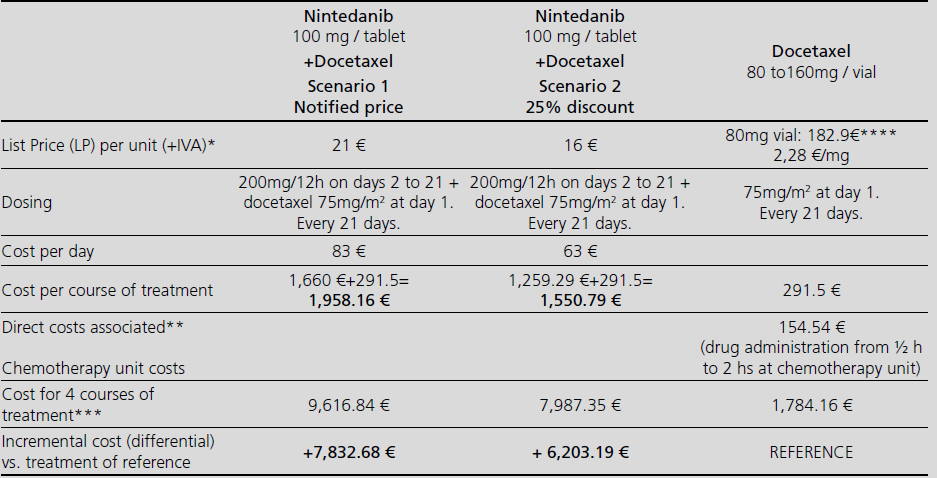
Nintedanib in combination with docetaxel for second-line treatment of advanced non-small-cell lung cancer; GENESIS-SEFH drug evaluation reporta