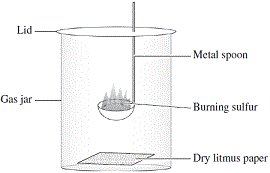
What is the nature (acidic/basic) of the following oxides (A) magnesium oxide (B) sulphur dioxide Give reason for - Science - Materials Metals and Non-Metals - 14184625 | Meritnation.com
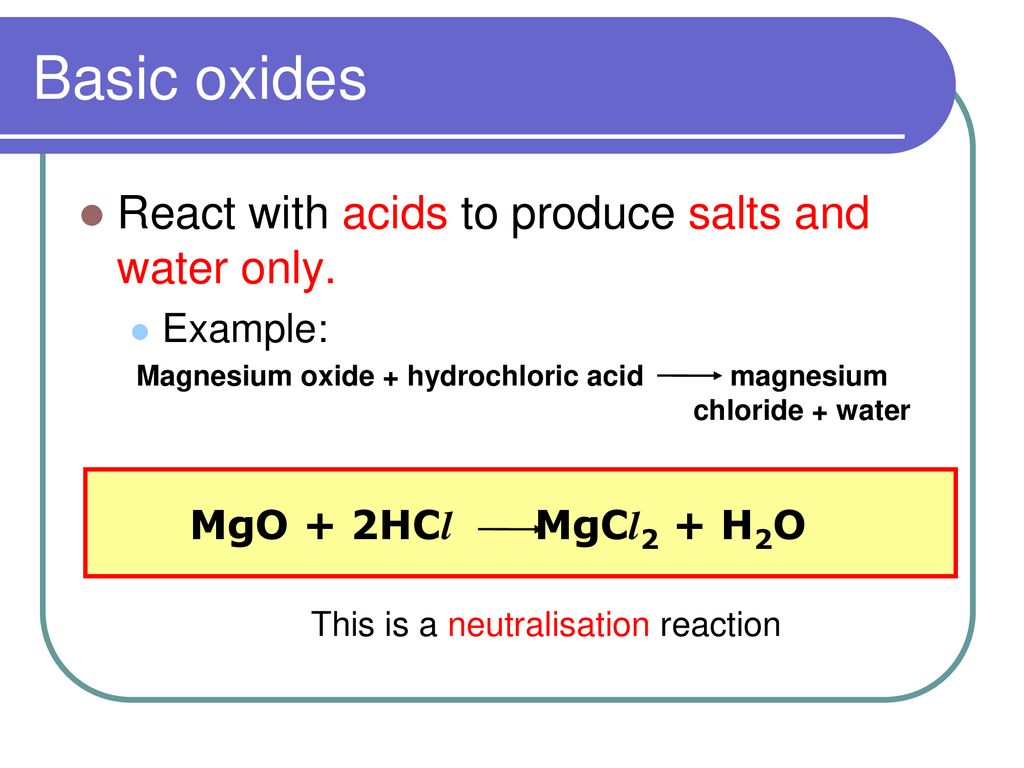
What is an oxide? An oxide is a Binary compound of oxygen and another element. M & O Oxides can be classified in two ways – Nature of Oxides Amount of. -

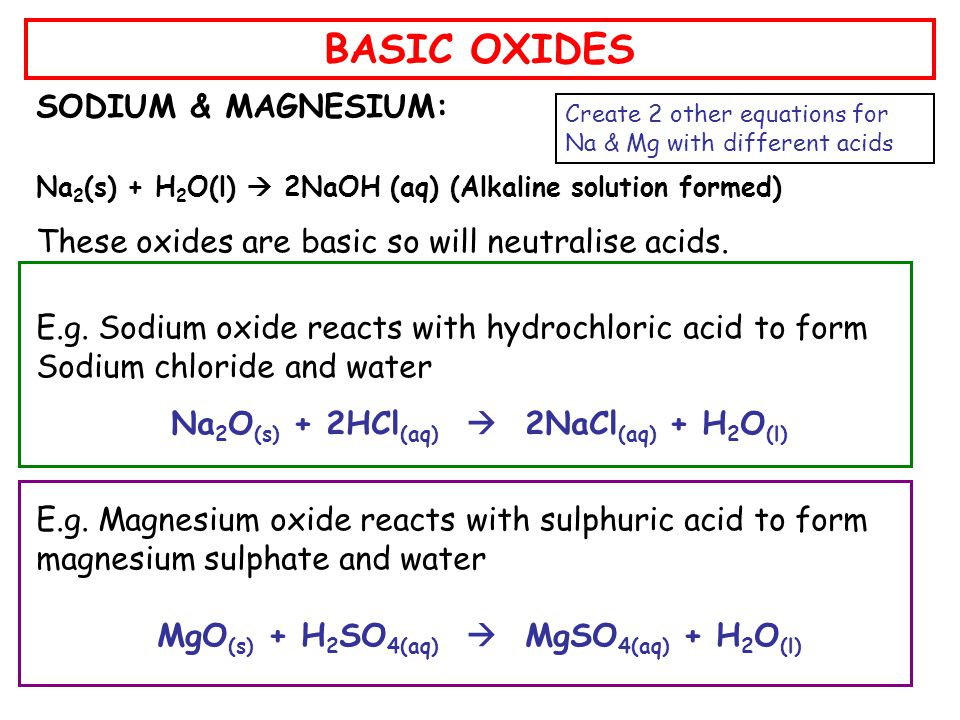
Generally, metallic oxides are basic and non - metallic oxides are acidic in nature. Solution of which of the following oxides in water will change the colour of blue litmus to red?
![Choose the most appropriate answer from the following list of oxides which fit the descriptions. Each answer may be used only once: [SO2, SiO2, Al2O3, MgO, CO, Na2O] (i) A basic oxide(ii) Choose the most appropriate answer from the following list of oxides which fit the descriptions. Each answer may be used only once: [SO2, SiO2, Al2O3, MgO, CO, Na2O] (i) A basic oxide(ii)](https://d1hhj0t1vdqi7c.cloudfront.net/v1/MzdncHQ0MGppZGc=/sd/)
Choose the most appropriate answer from the following list of oxides which fit the descriptions. Each answer may be used only once: [SO2, SiO2, Al2O3, MgO, CO, Na2O] (i) A basic oxide(ii)
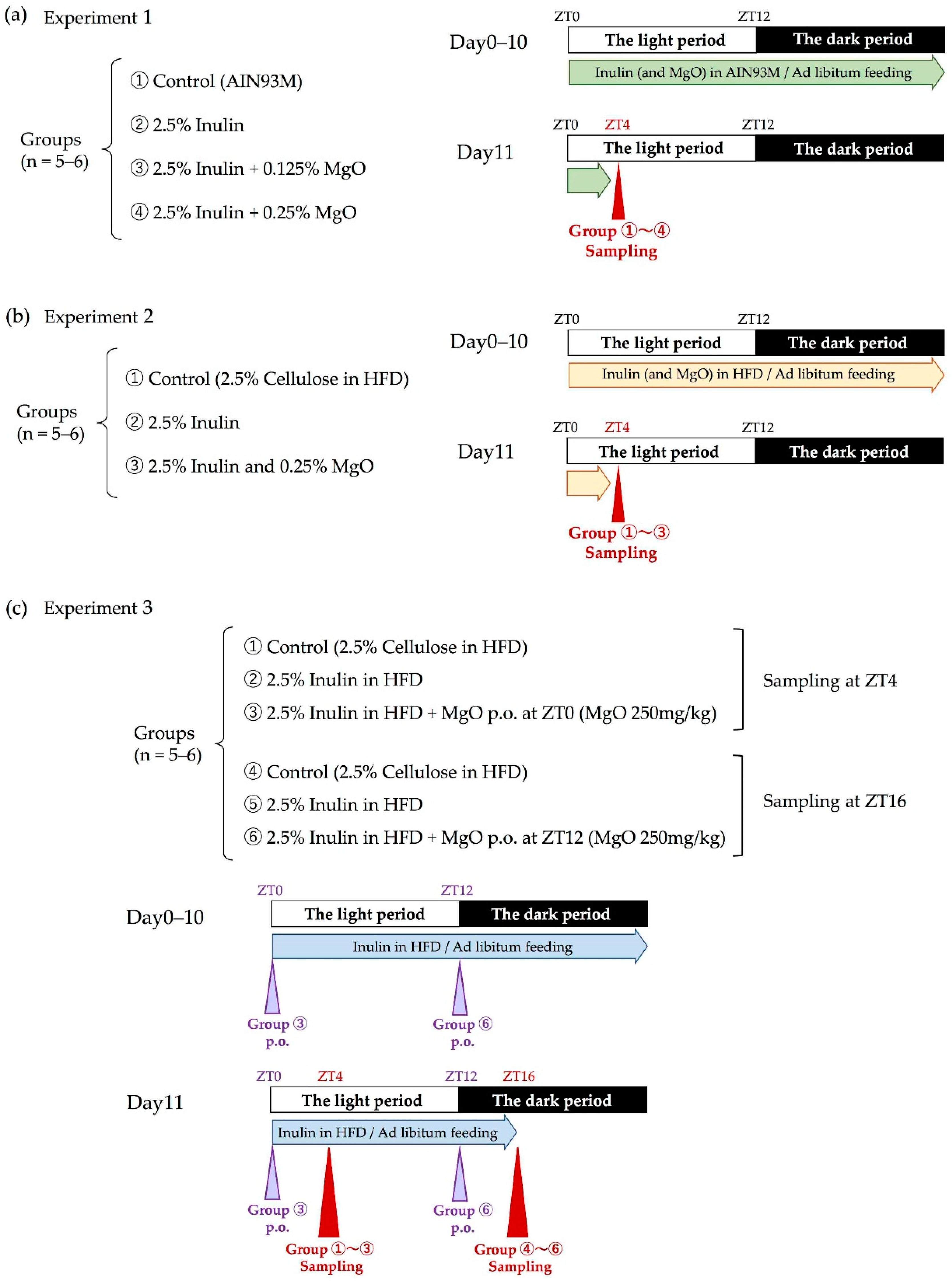
Nutrients | Free Full-Text | The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids



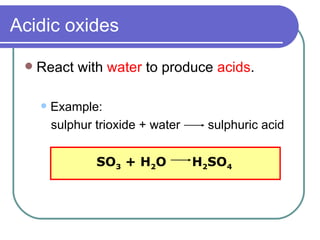
![SPM Chemistry Nature of Oxides] Chapter 4 - SPM - SKORA SPM Chemistry Nature of Oxides] Chapter 4 - SPM - SKORA](https://forum.spmpaper.me/uploads/default/original/1X/f1518bc68bed38126bbe3284c9f8c4895e3f1c60.jpeg)