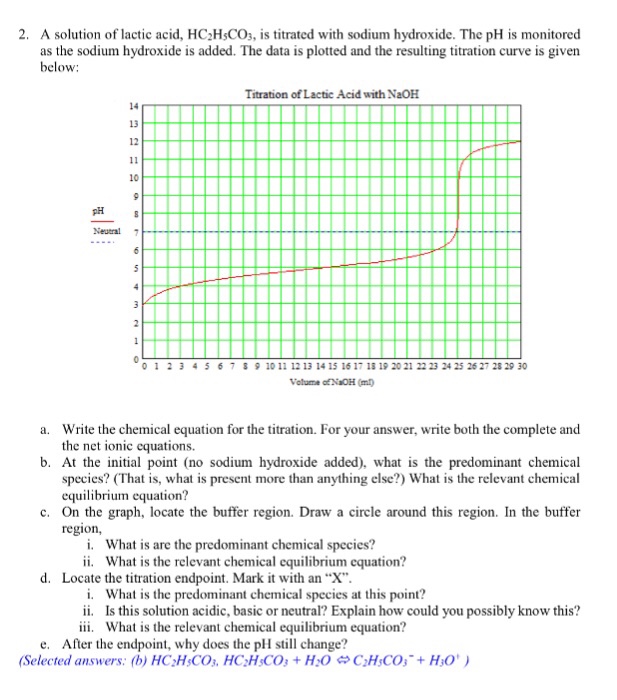
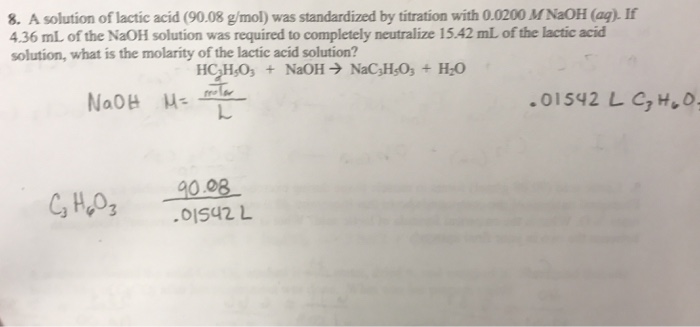SOLVED: Question 14 1pts 100 mLof 0.1 M NaOH is added to 55 mL of 0.2 M lactic acid. The pKa of lactic acid is 4.1. The resulting mixture has a pH close to: 6.9 0 5.1 0 8.3 0 44 Question 15
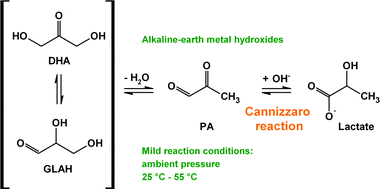
Synthesis of lactic acid from dihydroxyacetone: use of alkaline-earth metal hydroxides - Catalysis Science & Technology (RSC Publishing)

Selective Conversion of Glucose into Lactic Acid with Transition Metal Ions in Diluted Aqueous NaOH Solution | ACS Sustainable Chemistry & Engineering

SOLVED: Explain why your diluted solution of NaOH nccds to be standardized. Bc spccific: A25.00 mL samplc of0.3746 M lactic acid (C,H,OCOOH) requires 19.63 mL of 4 sodium hydroxide solution to reach

Selective Conversion of Glucose into Lactic Acid with Transition Metal Ions in Diluted Aqueous NaOH Solution | ACS Sustainable Chemistry & Engineering

Potentiometric titration of lactic acid. a The titration curve of 50 ml... | Download Scientific Diagram

If you wanted to hydrolyze 1 mole of PLA into 1 mole of sodium lactic acid, how many moles of sodium hydroxide would you need? | Homework.Study.com
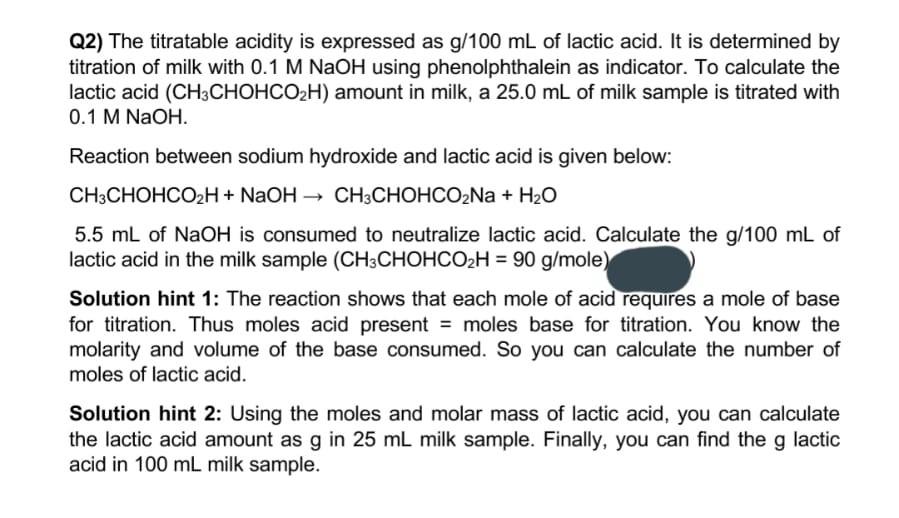
SOLVED: Note: Problem #Lis required Lactic acid builds up in the muscles when one exercises. 200 mL of 0.400 M lactic acid (HLac) was reacted with 100 mL of 0.500 M NaOH
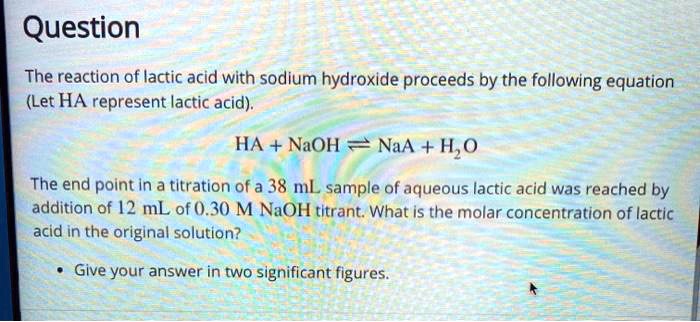
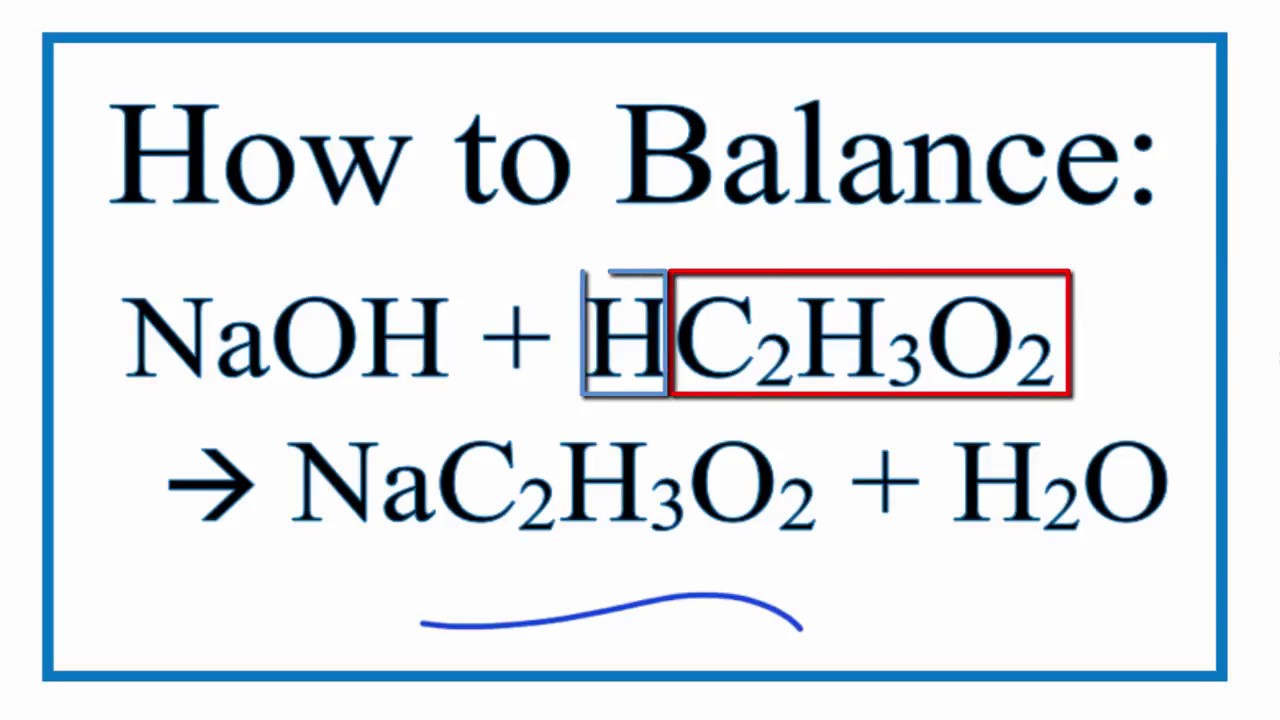
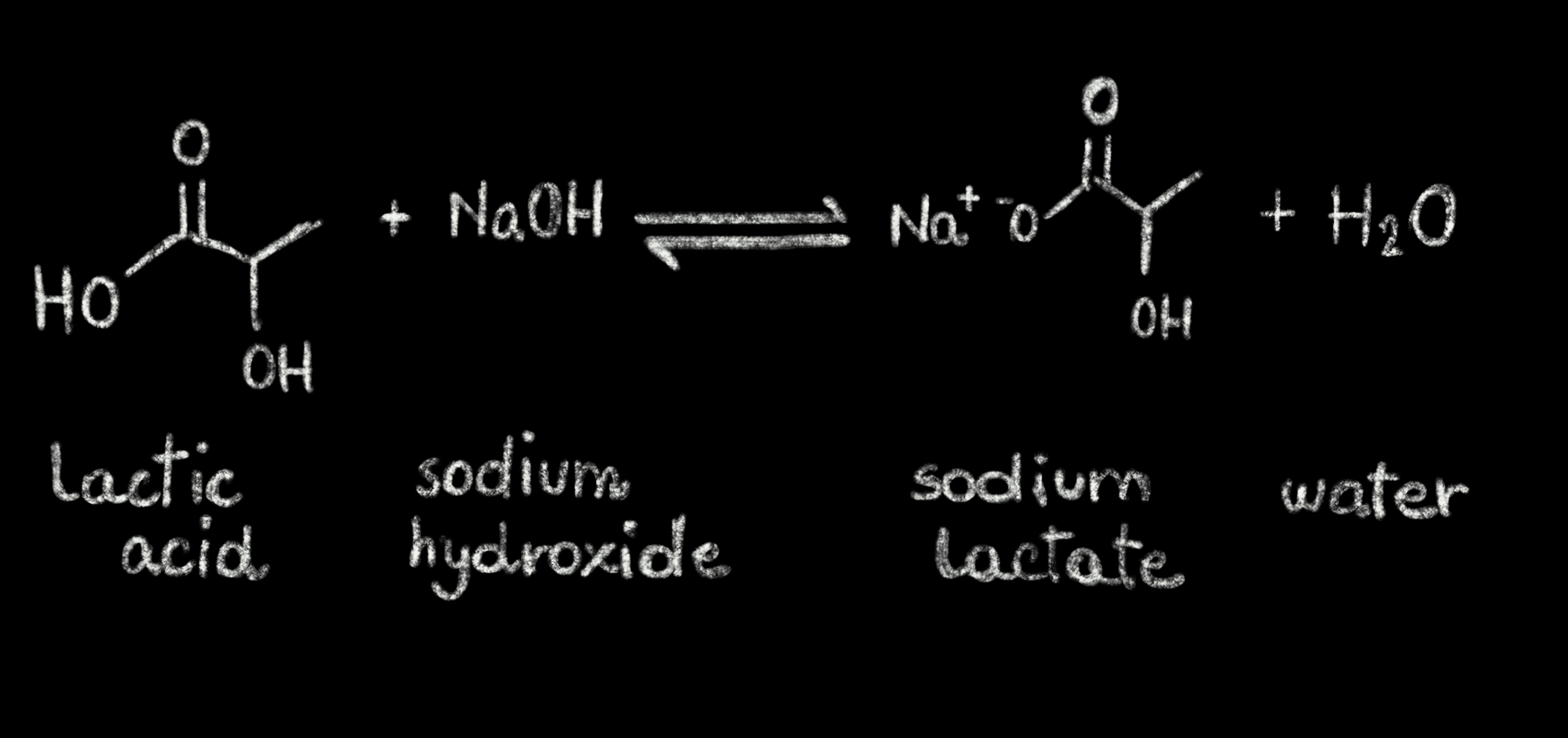
SOLVED: Question The reaction of lactic acid with sodium hydroxide proceeds by the following equation (Let HA represent lactic acid). HA + NaOH - NaA + H,O The end point In a
Lactic acid HC3H5O3 has one acidic hydrogen. A 0.10 M solution of lactic acid has the concentration of hydronium ion of 0.00363 M. Calculate Ka for lactic acid? | Socratic

pH titration curve of the aqueous lactic acid solution. The original... | Download Scientific Diagram
![SOLVED:How many microliters of 1.000 M NaOH solution must be added to 25.00 mL of a 0.1000 M solution of lactic acid [CH3 CH(OH) COOH. or .HC3 H5 O3] to produce a SOLVED:How many microliters of 1.000 M NaOH solution must be added to 25.00 mL of a 0.1000 M solution of lactic acid [CH3 CH(OH) COOH. or .HC3 H5 O3] to produce a](https://cdn.numerade.com/previews/a71cd3ce-5045-4f39-a5f1-44cb28587742.gif)
SOLVED:How many microliters of 1.000 M NaOH solution must be added to 25.00 mL of a 0.1000 M solution of lactic acid [CH3 CH(OH) COOH. or .HC3 H5 O3] to produce a

Hydrothermal conversion of glucose into lactic acid with sodium silicate as a base catalyst - ScienceDirect

Scheme 1. Reagents and conditions: (a) lactic acid, 4 M HCl, reflux;... | Download Scientific Diagram