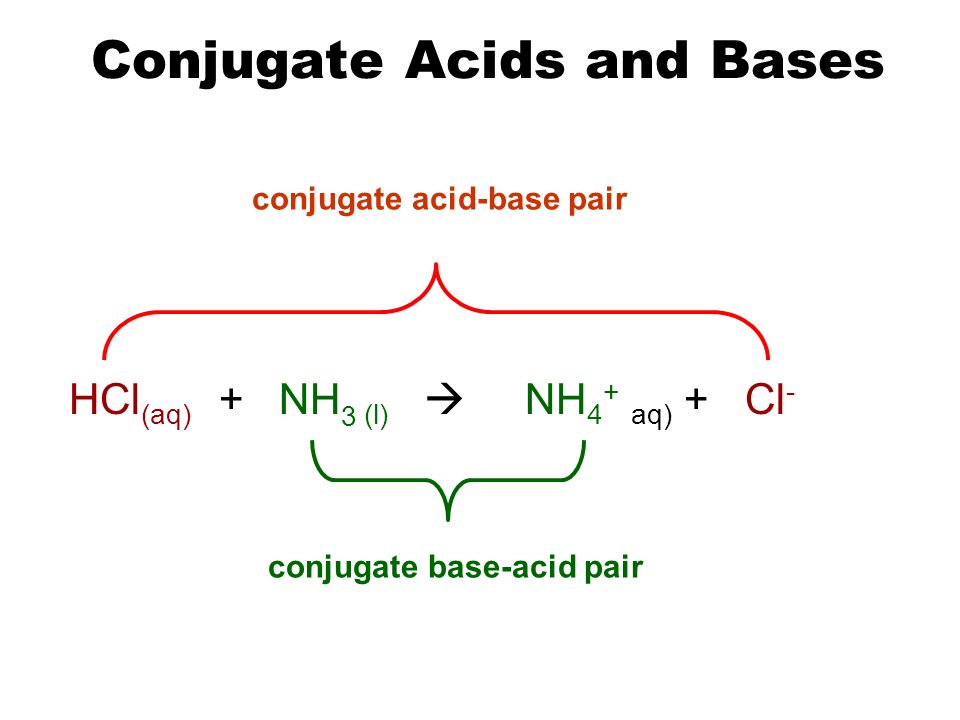
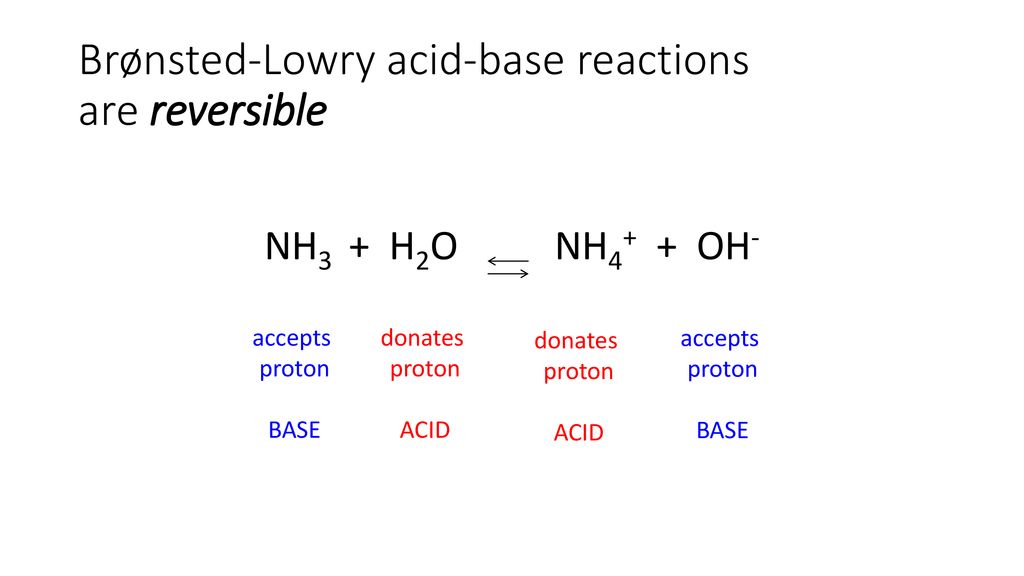
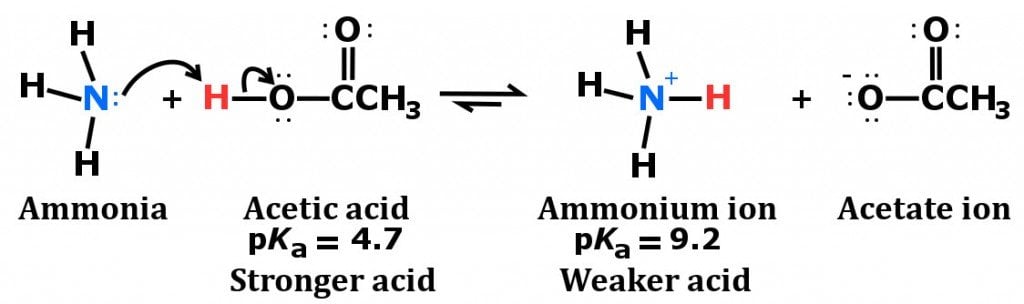
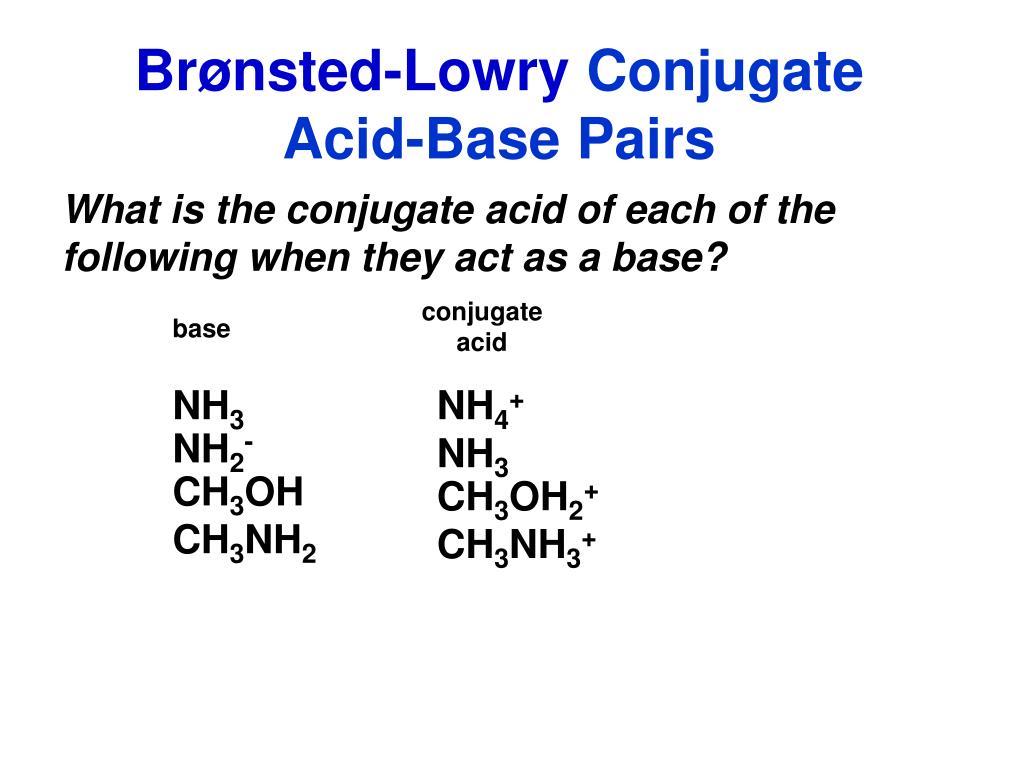
organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange

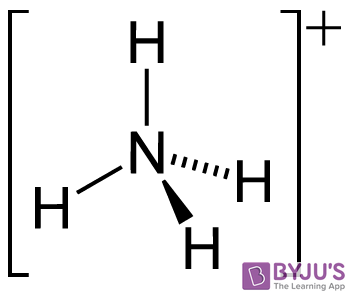
Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com
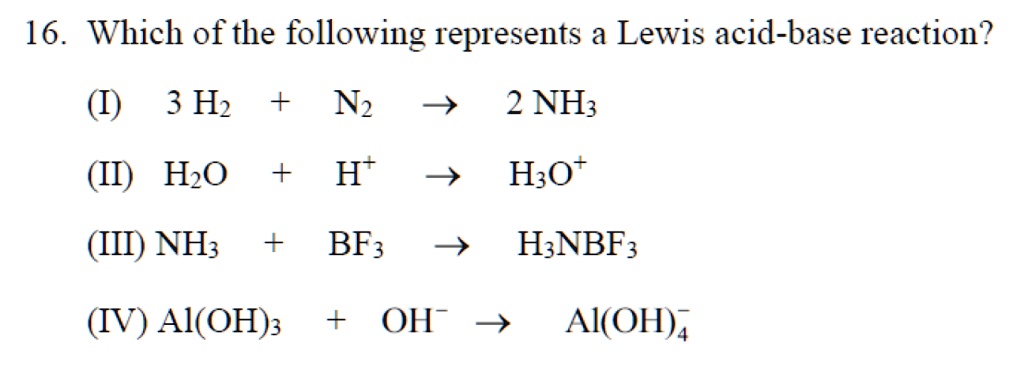
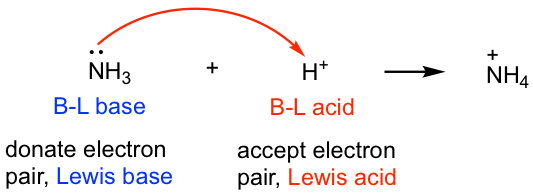
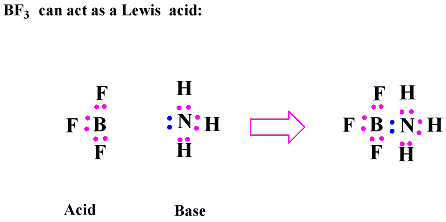
SOLVED: 16. Which of the following represents a Lewis acid-base reaction? (I) 3 Hz + N2 2 NH3 (II) Hzo + H H;ot (III) NH; 1 BF3 HNBF; (IV) AI(OH)3 T OH- AI(OH)A

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in