![Among the following, which complex compound is diamagnetic?i) [Co(NH3)6]Cl3 ,ii) [Ni(NH3)6]Cl2 ,iii) [Cr(H2O)6]Cl3 ,iv) [Fe(H2O)6]Cl2 Among the following, which complex compound is diamagnetic?i) [Co(NH3)6]Cl3 ,ii) [Ni(NH3)6]Cl2 ,iii) [Cr(H2O)6]Cl3 ,iv) [Fe(H2O)6]Cl2](https://d1hhj0t1vdqi7c.cloudfront.net/v1/V0FNUzRla0szUnM=/sd/)
Among the following, which complex compound is diamagnetic?i) [Co(NH3)6]Cl3 ,ii) [Ni(NH3)6]Cl2 ,iii) [Cr(H2O)6]Cl3 ,iv) [Fe(H2O)6]Cl2
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1280/0*8wwL8Ru43LyLIIL8.png)
Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

The calculated molar absorbance spectra for nickel species (Ni 2+ ,... | Download Scientific Diagram

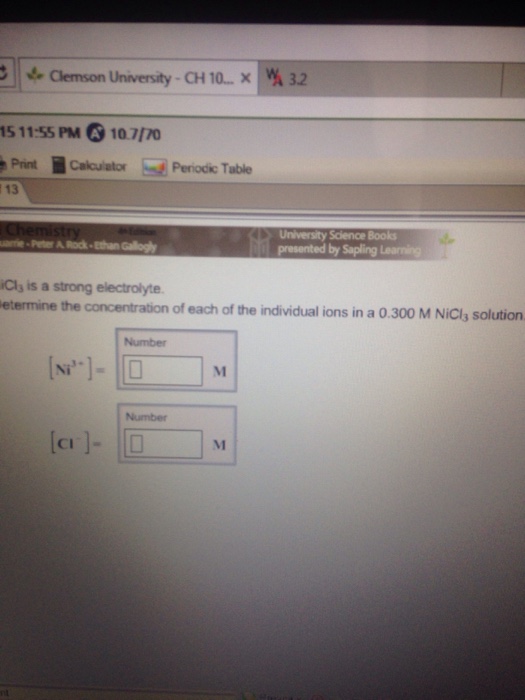
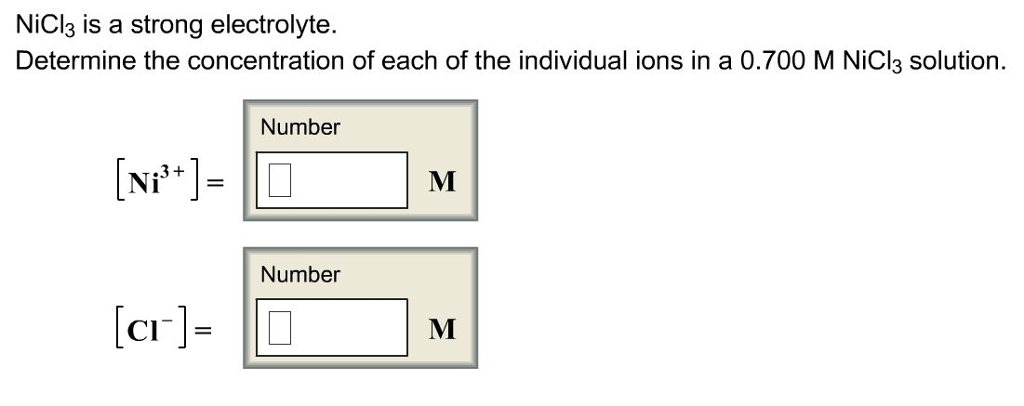
OneClass: NiCl3 is a strong electrolyte. Determine the concentration ofeach of the individual ions in...

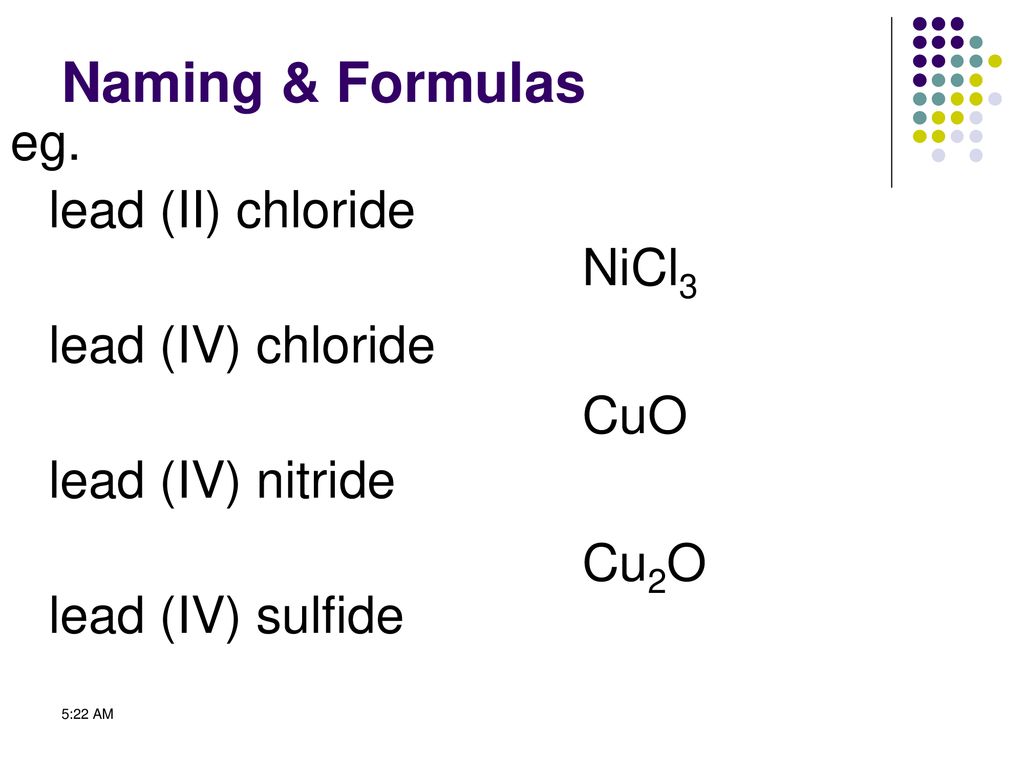
Ions Atoms that lose electrons (negative) have more positive charge than negative charge so they are positive ions. Atoms that gain electrons (negative) - ppt download

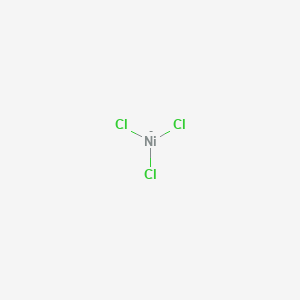
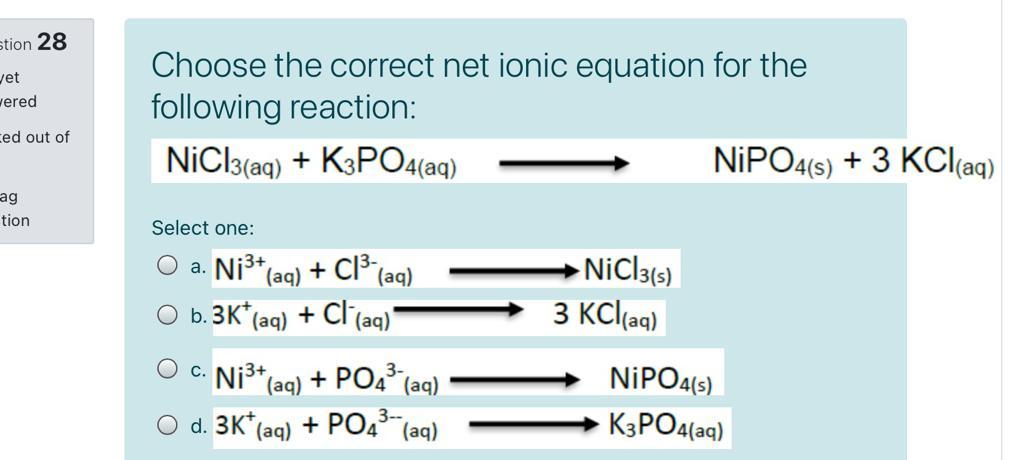
SOLVED: How many Ampers (A) are needed to deposit 0.4685 g of Ni metal from an aqueous solution of NiCl3, in 495 seconds?
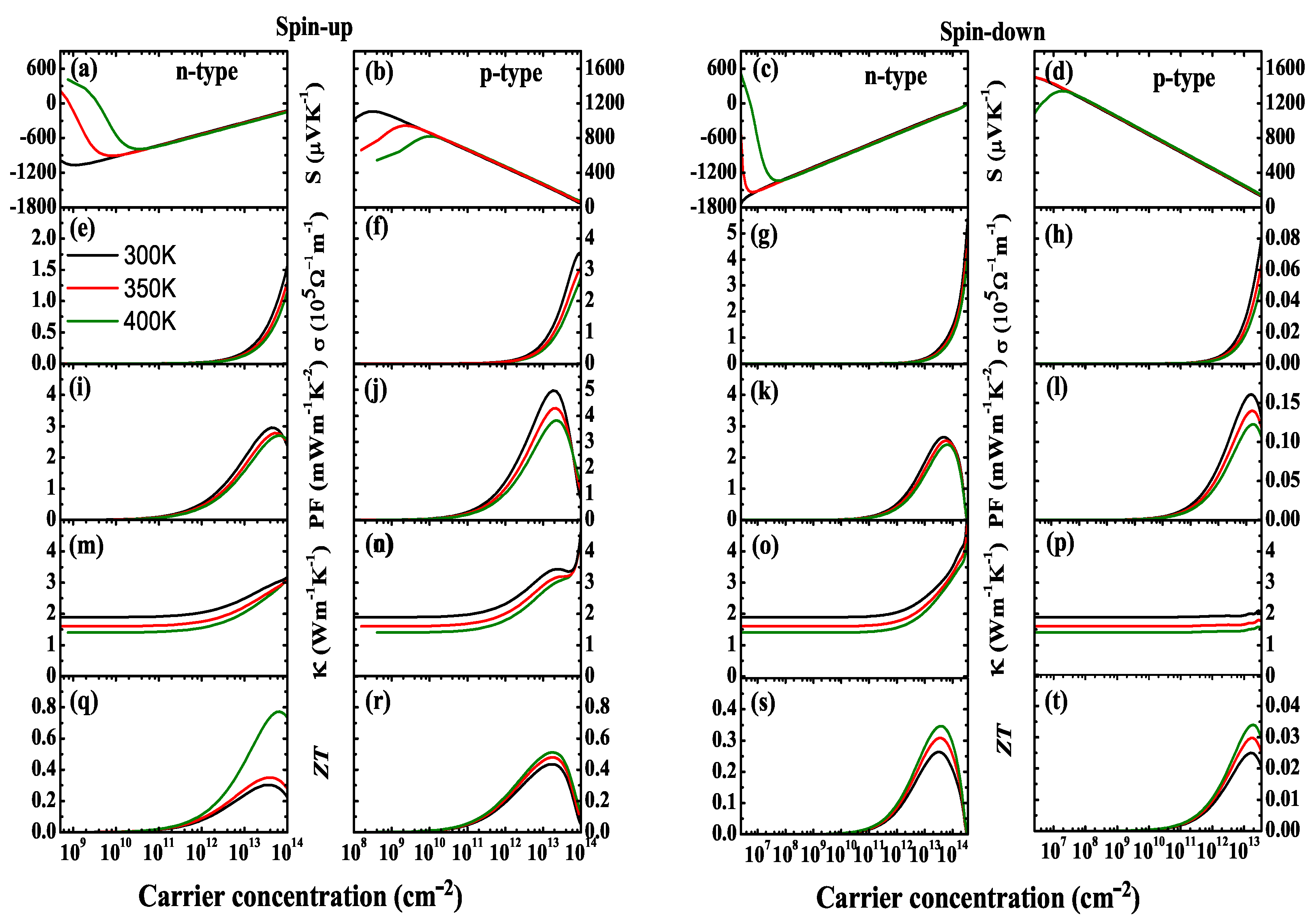
Nanomaterials | Free Full-Text | Thermoelectric Properties of NiCl3 Monolayer: A First-Principles-Based Transport Study
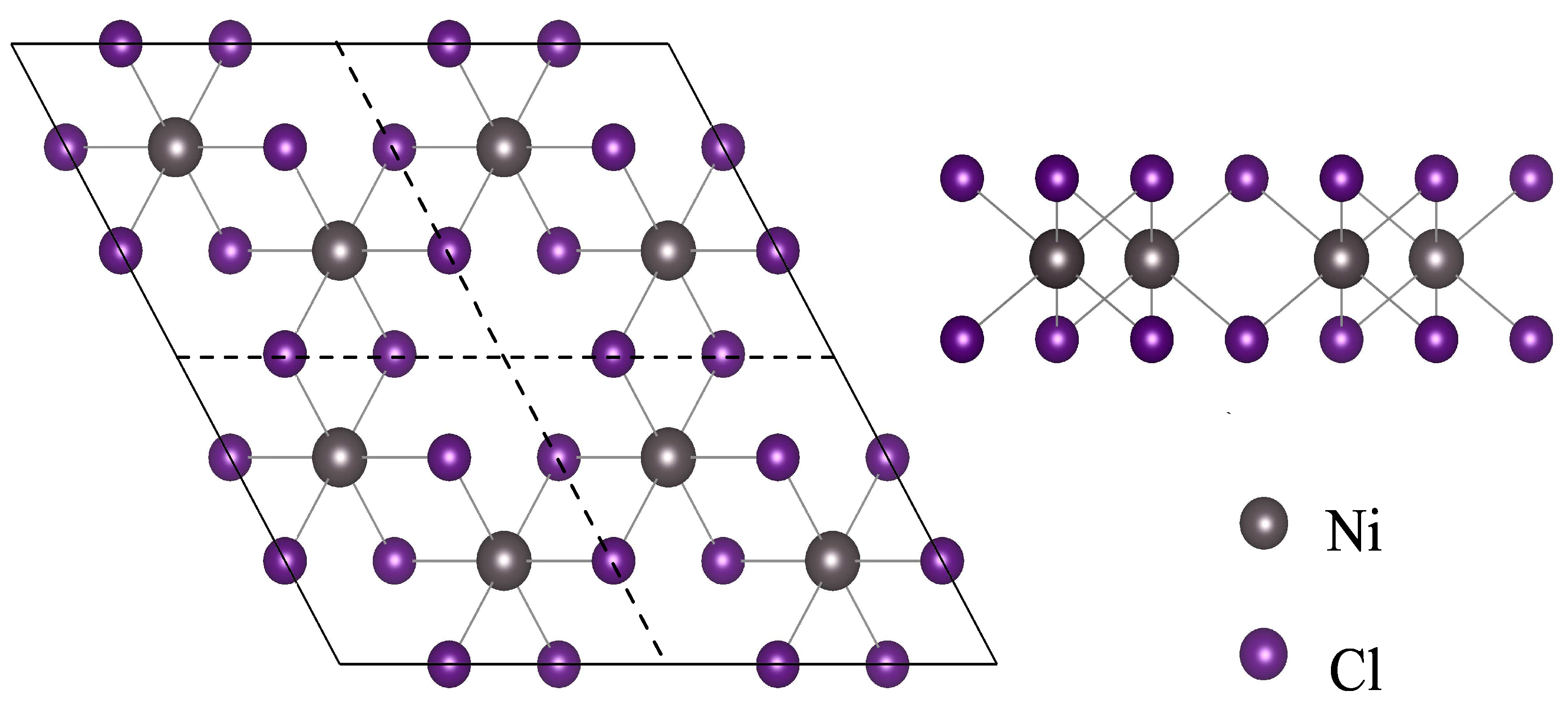
Nanomaterials | Free Full-Text | Thermoelectric Properties of NiCl3 Monolayer: A First-Principles-Based Transport Study
![FTIR spectra of a Ni(Sal)2, b [Cr(en)3]Cl3.3H2O, and c NiCr2O4 (sample 2) | Download Scientific Diagram FTIR spectra of a Ni(Sal)2, b [Cr(en)3]Cl3.3H2O, and c NiCr2O4 (sample 2) | Download Scientific Diagram](https://www.researchgate.net/publication/313686161/figure/fig1/AS:941596942024714@1601505613109/FTIR-spectra-of-a-NiSal2-b-Cren3Cl33H2O-and-c-NiCr2O4-sample-2.gif)
FTIR spectra of a Ni(Sal)2, b [Cr(en)3]Cl3.3H2O, and c NiCr2O4 (sample 2) | Download Scientific Diagram
![Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number](https://i.pinimg.com/736x/cb/55/b9/cb55b933ee9ec4d59fdf9d226cf3a51a.jpg)
Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1090/0*rkWRh7JsXBvtOfdK.jpg)
Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
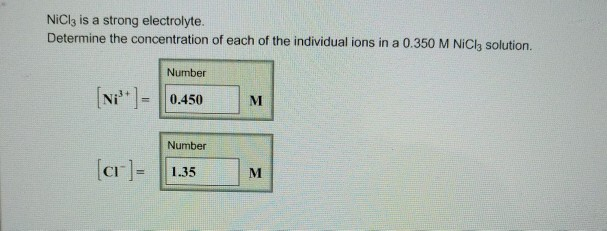
![SOLVED: NiCl3 is a strong electrolyte. Determine the concentration of each of the individual ions in a 0.400 M NiCl3 solution. [Ni3+]= [ Ni 3 + ] = M M [Cl−]= SOLVED: NiCl3 is a strong electrolyte. Determine the concentration of each of the individual ions in a 0.400 M NiCl3 solution. [Ni3+]= [ Ni 3 + ] = M M [Cl−]=](https://cdn.numerade.com/ask_previews/27b35ed4-efd2-4a05-861f-28a8ce34d98b_large.jpg)


![OneClass: What are all the isomers of [NiCl3(H2O)][PF6]? OneClass: What are all the isomers of [NiCl3(H2O)][PF6]?](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/121/12105789.png)