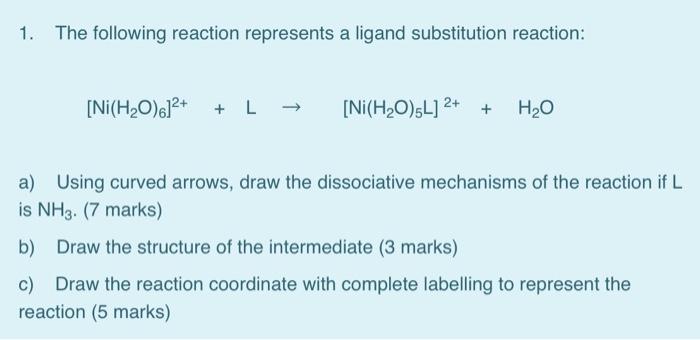
![A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp](https://external-preview.redd.it/VDYxIhyF0tX1EvHO09Izi030EWZe0sji-zLW3q5xn38.jpg?auto=webp&s=05f1b2c012b7b91e46340d66d18806bcaa6e97b2)
A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp
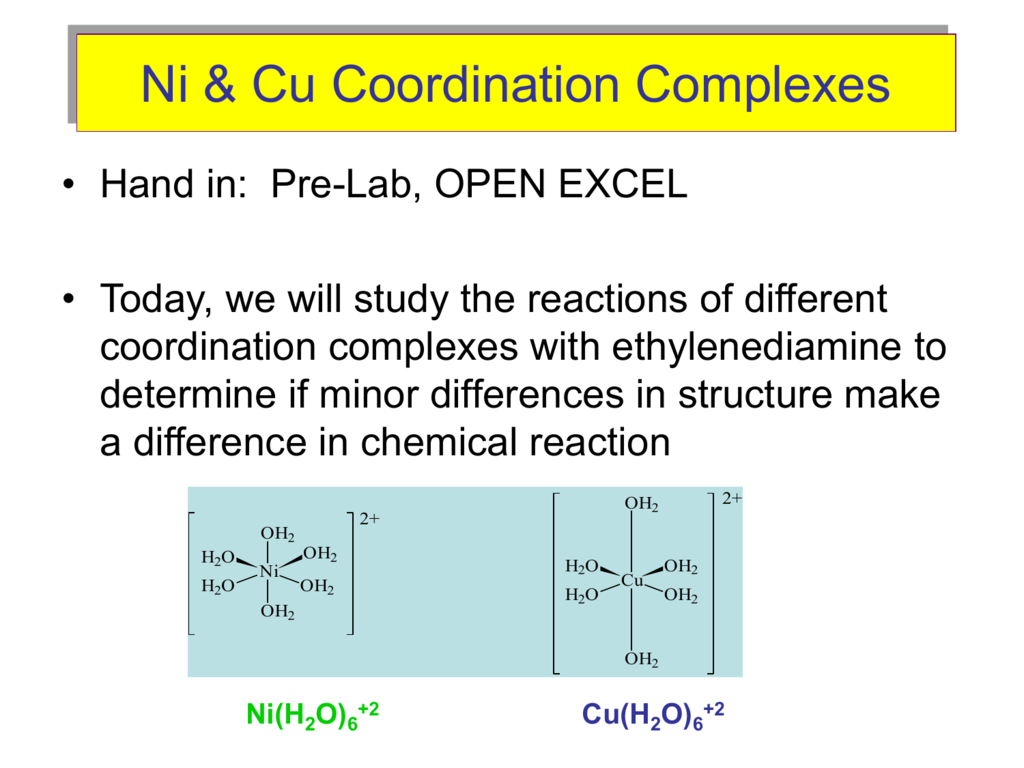
a. [Ni(H2O)6]^2+ (aq) is green in colour whereas [Ni(H2O)4 (en)^2+ (aq)is blue in colour , give reason in support of your answer . - Sarthaks eConnect | Largest Online Education Community
2. All atoms, except the | Download Scientific Diagram Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig5/AS:667921089052683@1536256205687/Representation-of-the-unit-cell-of-NiH2O6NO32-All-atoms-except-the.jpg)
Representation of the unit cell of [Ni(H2O)6](NO3)2. All atoms, except the | Download Scientific Diagram
✓ Solved: Describe the distribution of d electrons in [Ni(H2O)6]^2+, using crystal field theory. How...
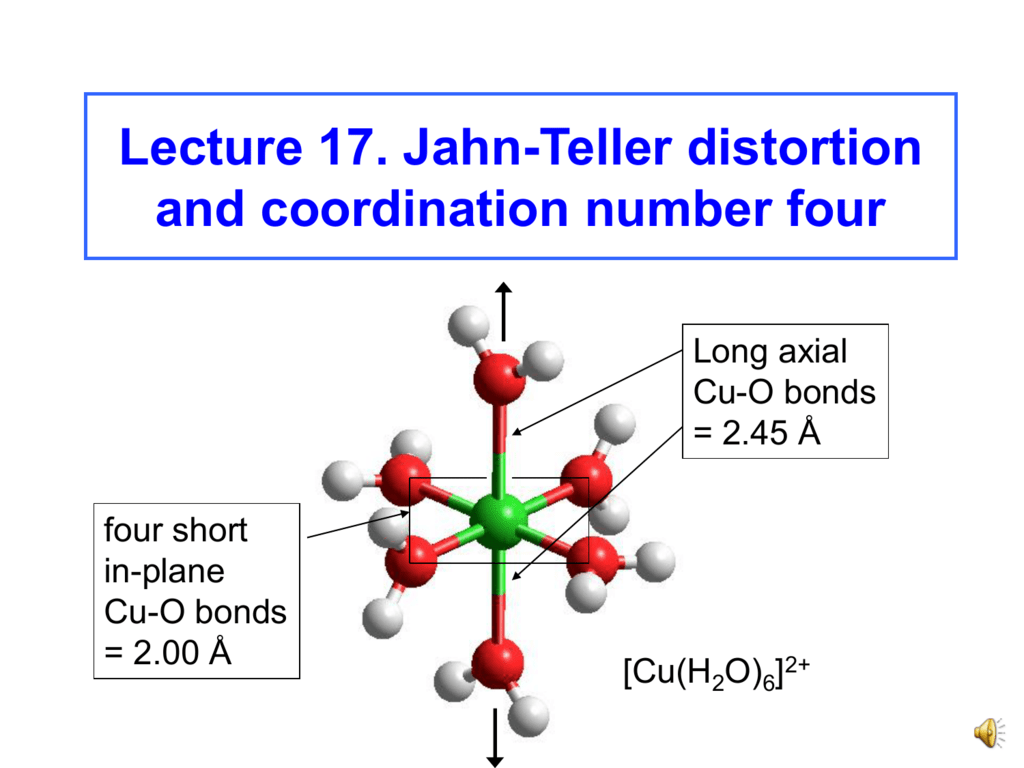
![Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic0010860/asset/images/large/ic0010860n00001.jpeg)
Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry
![Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_599e6db7e641a.png)
Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx2.jpg)
Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect
The hybridizations of Ni(CO)4 and Cr(H2O)6^2+, respectively, are - Sarthaks eConnect | Largest Online Education Community
Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][




![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://d1hhj0t1vdqi7c.cloudfront.net/v1/SFk3ZV9DMWxyZ28=/sd/)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure -Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure -Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)

![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://haygot.s3.amazonaws.com/questions/1955044_1567628_ans_f710a7dfc21d4ea7a5d920b41112c727.jpg)

