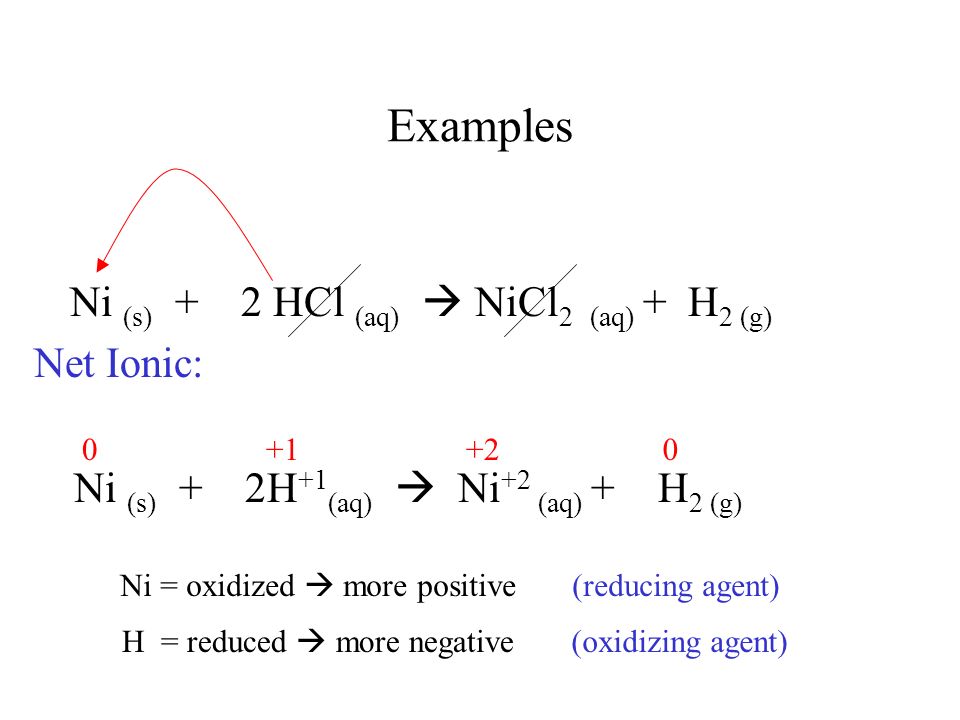
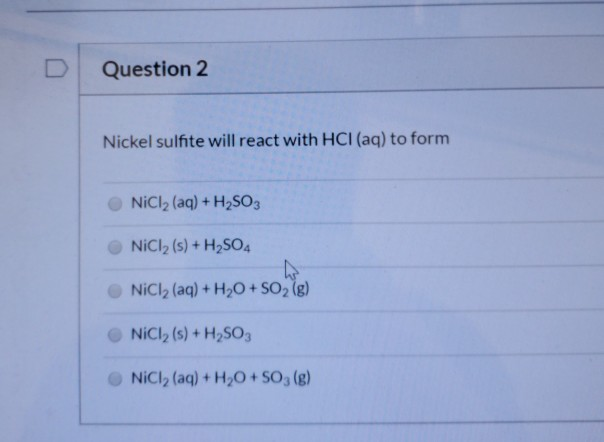
![A given nitrogen containing aromatic compound A reacts with \\[{\\text{Sn\/ HCl}}\\], followed by \\[{\\text{HN}}{{\\text{O}}_{\\text{2}}}\\] to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with molecular A given nitrogen containing aromatic compound A reacts with \\[{\\text{Sn\/ HCl}}\\], followed by \\[{\\text{HN}}{{\\text{O}}_{\\text{2}}}\\] to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with molecular](https://www.vedantu.com/question-sets/b565ff56-4b65-4f02-a304-a282cc9558db4662046607693484074.png)
A given nitrogen containing aromatic compound A reacts with \\[{\\text{Sn\/ HCl}}\\], followed by \\[{\\text{HN}}{{\\text{O}}_{\\text{2}}}\\] to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with molecular

Cr/Co/Cu/Fe/ Mn/Ni, HCL, Lumina, NO TIMER, 4-pin, Coded - Cableless, PerkinElmer N3050217 compatible

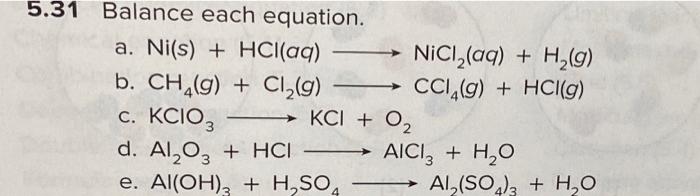
a) Draw the mechanism for the reaction of tin/HCl with m-nitroacetophenone. b) Why is sodium hydroxide added to the reaction? What tin compounds are produced? How do you separate the tin salts
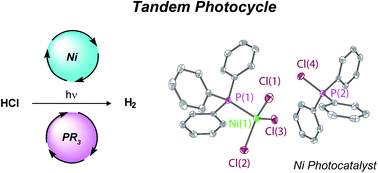
Tandem redox mediator/Ni(ii) trihalide complex photocycle for hydrogen evolution from HCl - Chemical Science (RSC Publishing)