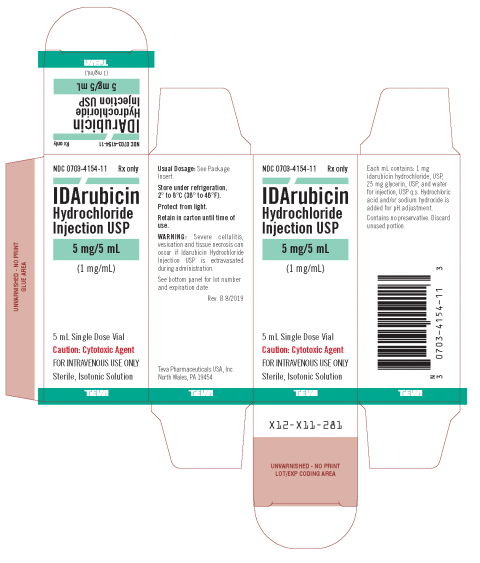
Teva Issues Voluntary Nationwide Recall of One Lot of IDArubicin Hydrochloride Injection USP 5 mg/5 mL Due to the Presence of Particulate Matter | FDA

Teva faces drug price fixing charges, to cut 350 jobs in Israel; Wintac gets hit by FDA Warning Letter | Radio Compass Blog