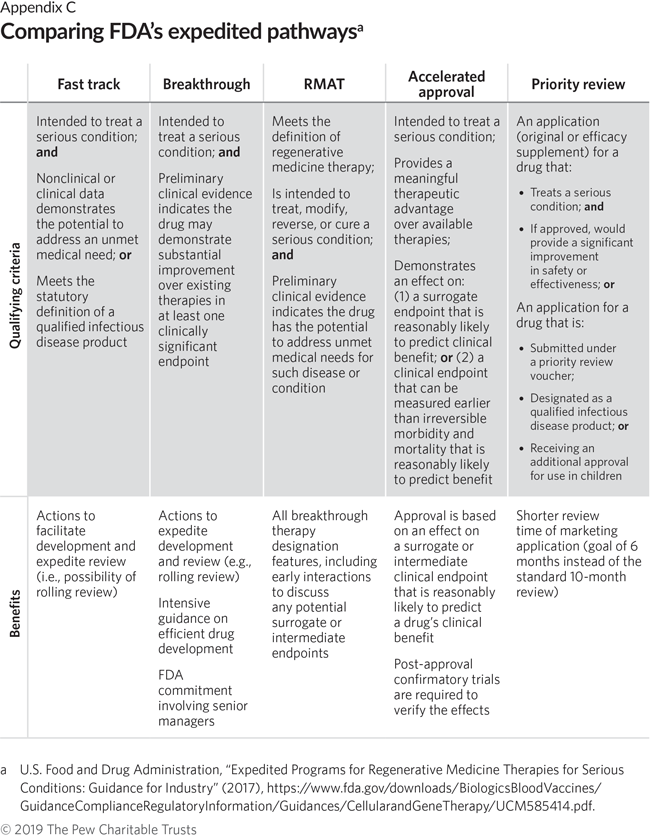
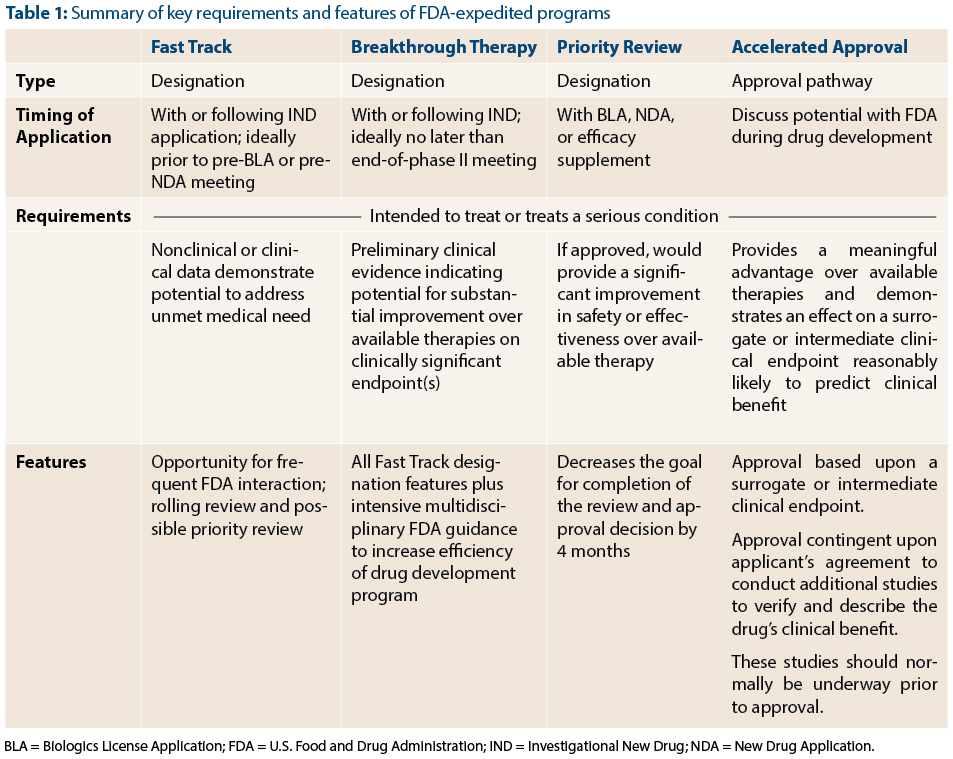
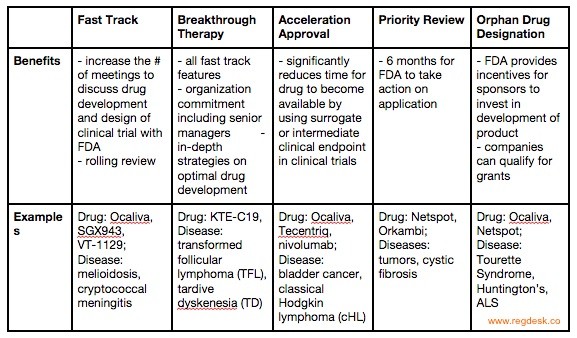
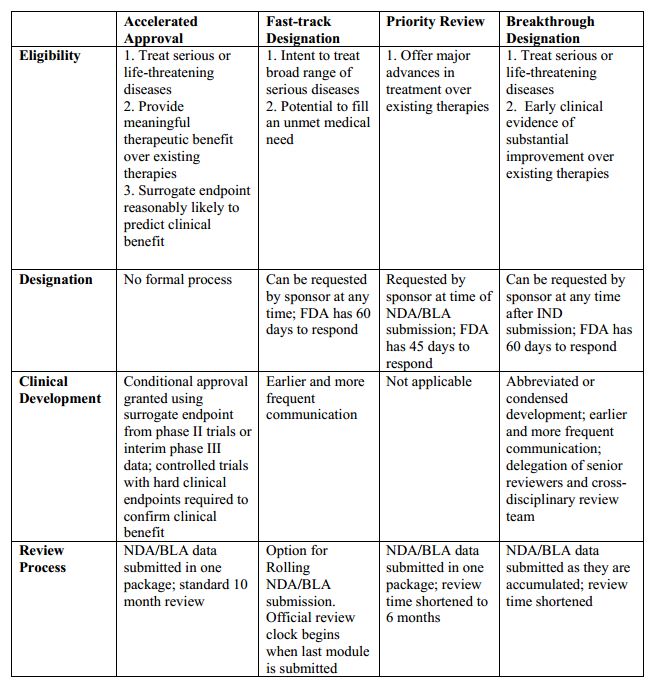
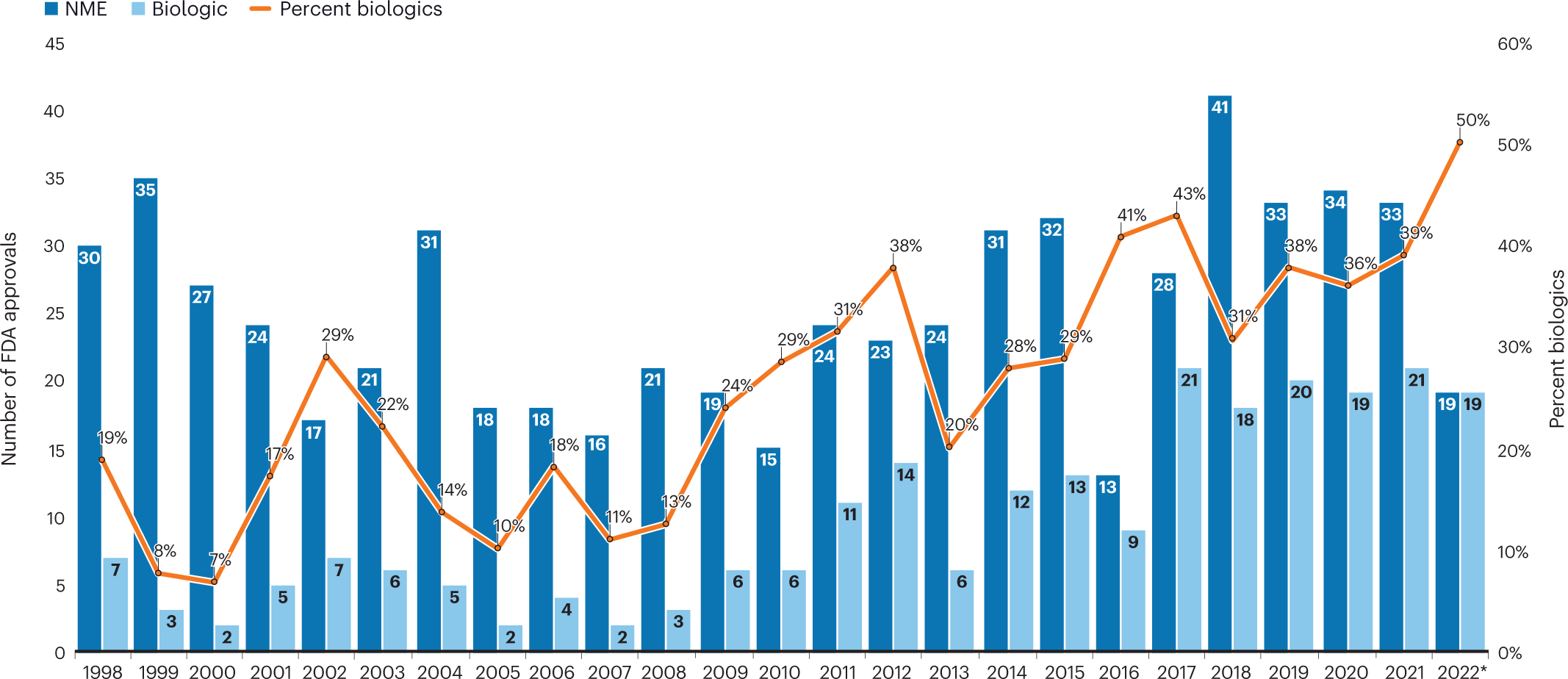
A detailed analysis of expedited regulatory review time of marketing authorization applications for new anticancer drugs in the US and EU - da Costa Gonçalves - 2022 - Clinical and Translational Science - Wiley Online Library
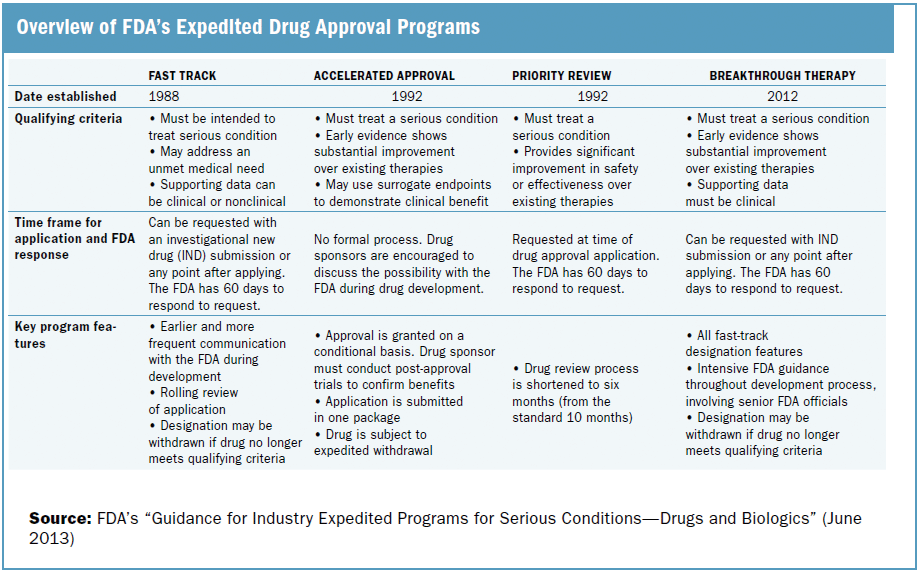
Development and Regulation of Medical Countermeasures for COVID-19 (Vaccines, Diagnostics, and Treatments): Frequently Asked Questions - EveryCRSReport.com
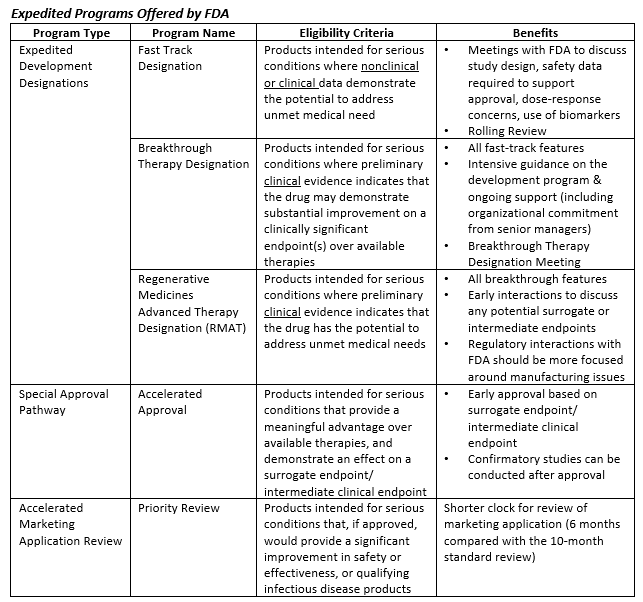
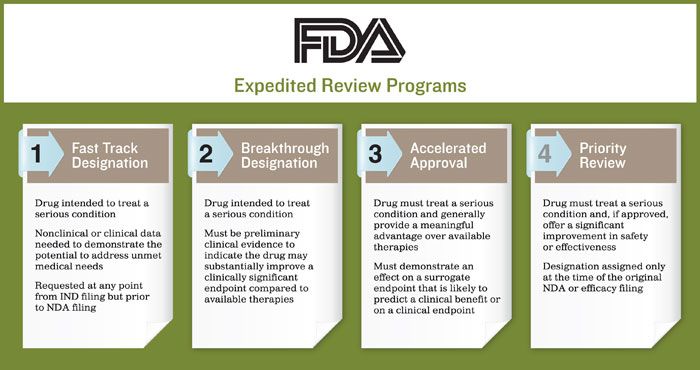
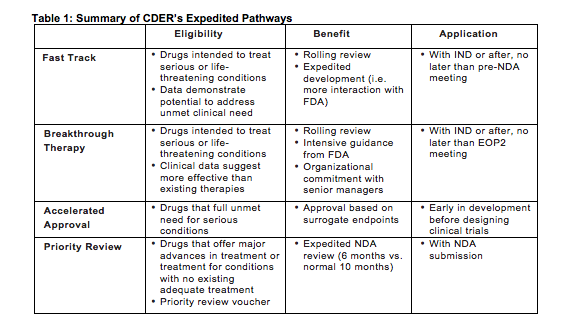
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group