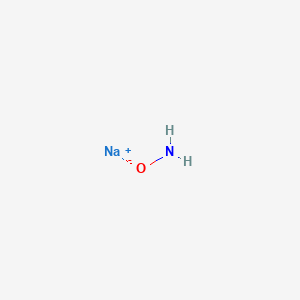
Two substances with empirical formula HNO are hyponitrous acid (M = 62.04 g/mol) and nitroxyl (M = - Brainly.com

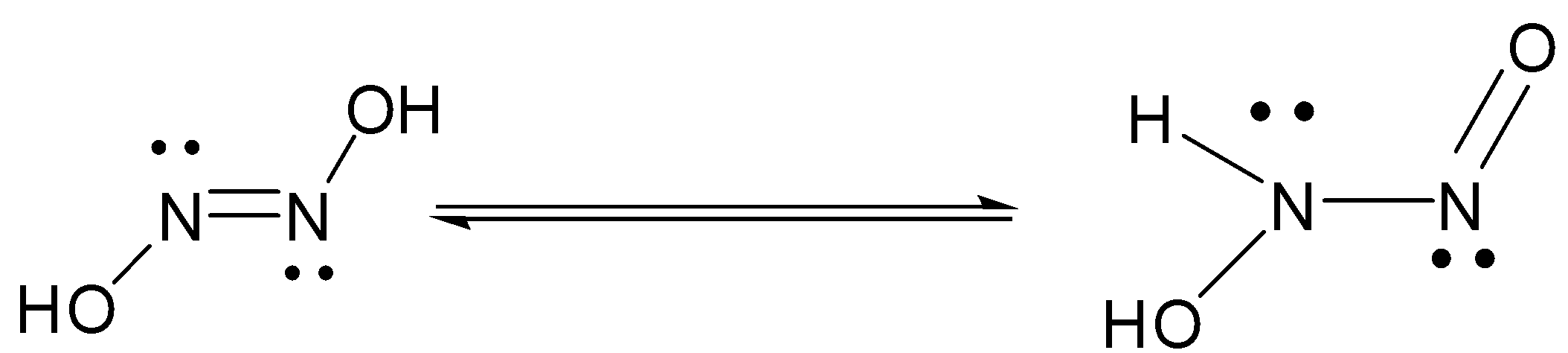
799. The mechanism of the oxidation of nitrous acid by hyponitrous acid. Part I - Journal of the Chemical Society (Resumed) (RSC Publishing)
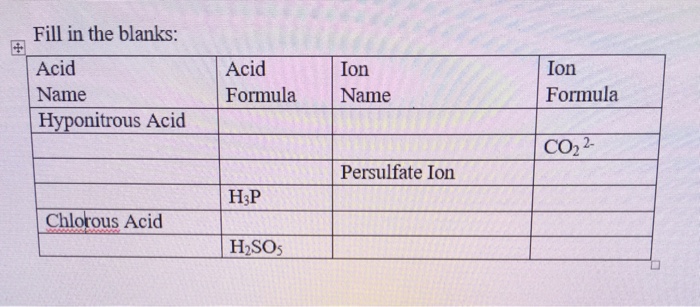
Solved: Chapter 21 Problem 101AP Solution | Onekey Blackboard, General Chemistry 4th Edition | Chegg.com
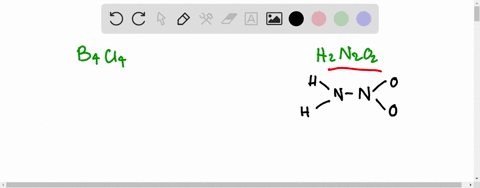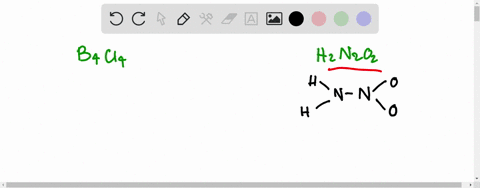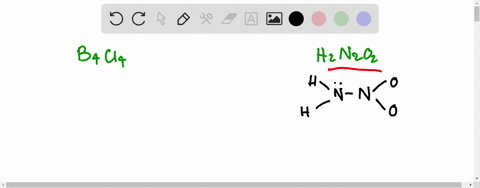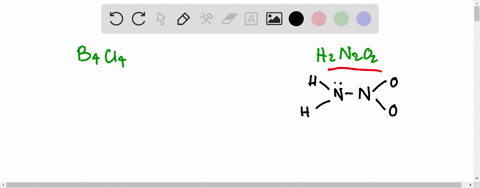
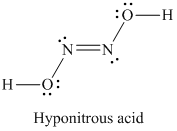
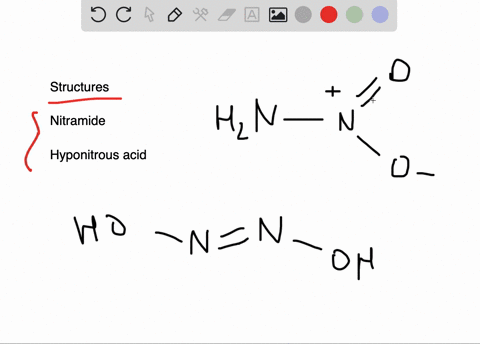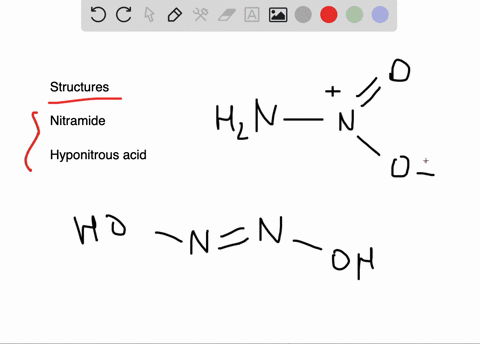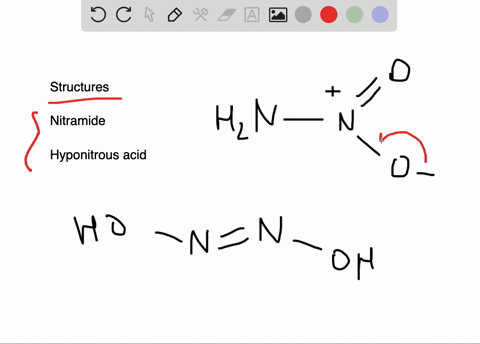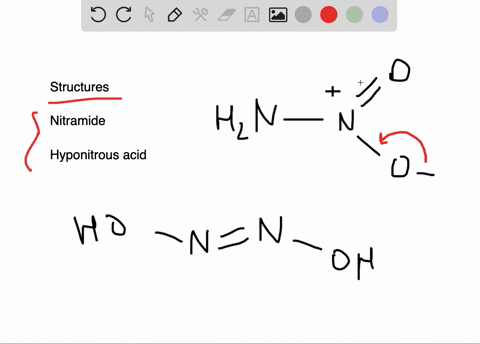
SOLVED:Write the Lewis structure for: (a) tetraboron tetrachloride, B4 Cl4 (b) hyponitrous acid, H2 N2 O2
Give the formula and the oxidation state of nitrogen in the following acids. (i) Hyponitrous acid, (ii) Nitrous acid, - Sarthaks eConnect | Largest Online Education Community

. Essentials of medical electricity . tion of sulphuric acidupon the zinc and transferred to the platinum element meetsthe nitric acid and decomposes it; hyponitrous acid is formedand passes off in fumes, constituting one of its chief objec-tions. The platinum is ...

Molecular graphs of the different conformers of hyponitrous acid and... | Download Scientific Diagram
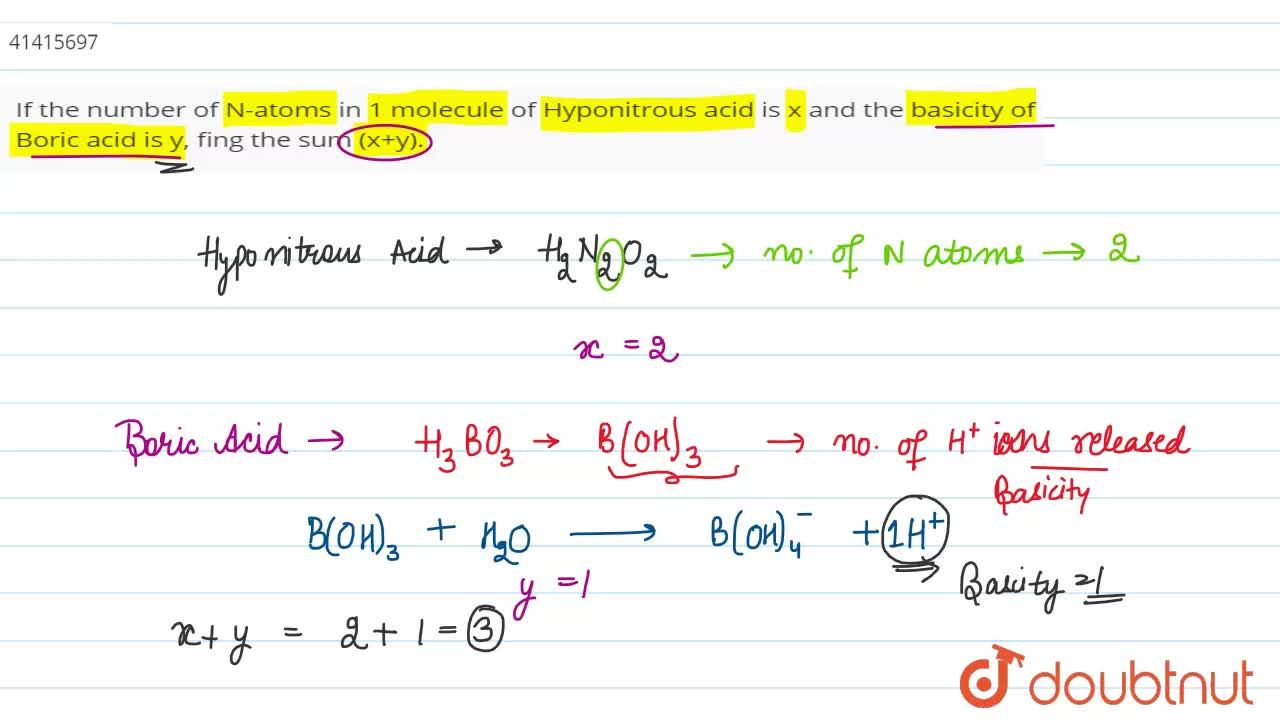
If the number of N-atoms in 1 molecule of Hyponitrous acid is x and the basicity of Boric acid is y, fing the sum (x+y).





![Hyponitrous acid] Hyponitrous acid]](https://www.degruyter.com/document/doi/00.0000/IUPAC.iupac.compound.61744/asset/images/61744.png)