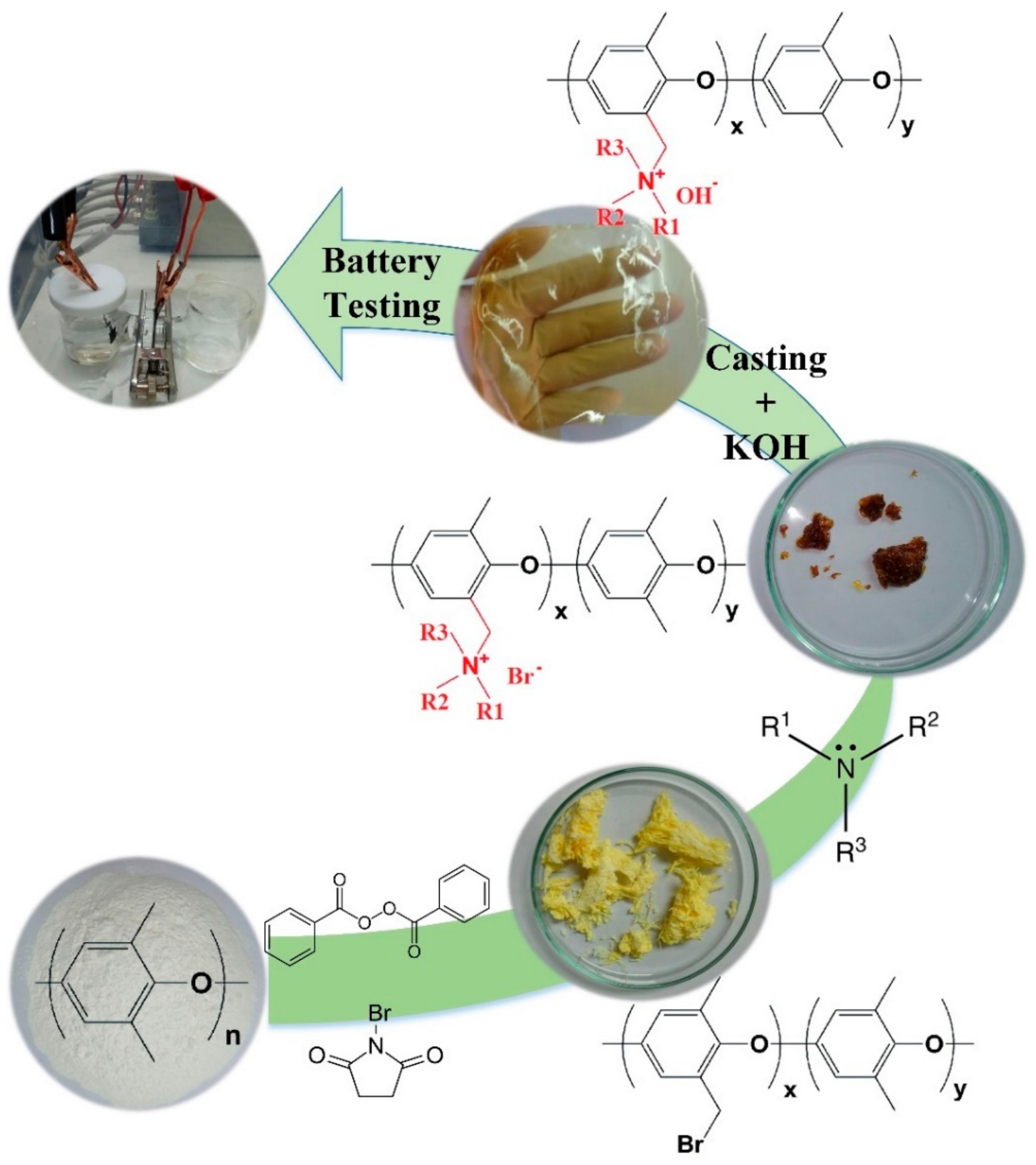
IJMS | Free Full-Text | Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery

An Accurate Molecular Structure of Phenyl, the Simplest Aryl Radical - Martinez - 2015 - Angewandte Chemie International Edition - Wiley Online Library

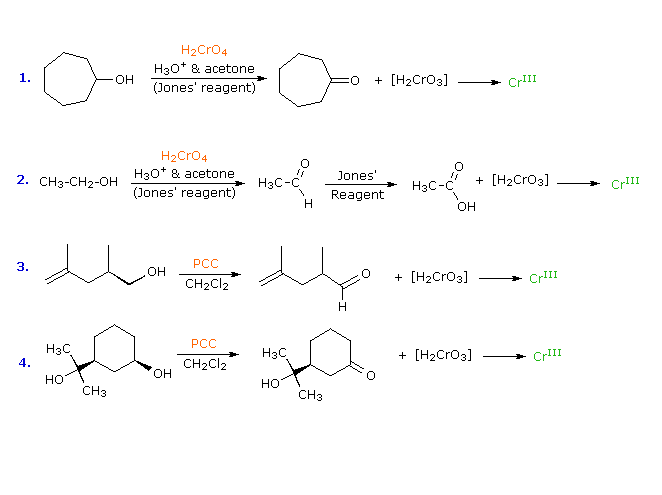
Answer the following:Although phenoxide ion has more number of resonating structures than benzoate ion, benzoic acid is stronger acid than phenol. Why?
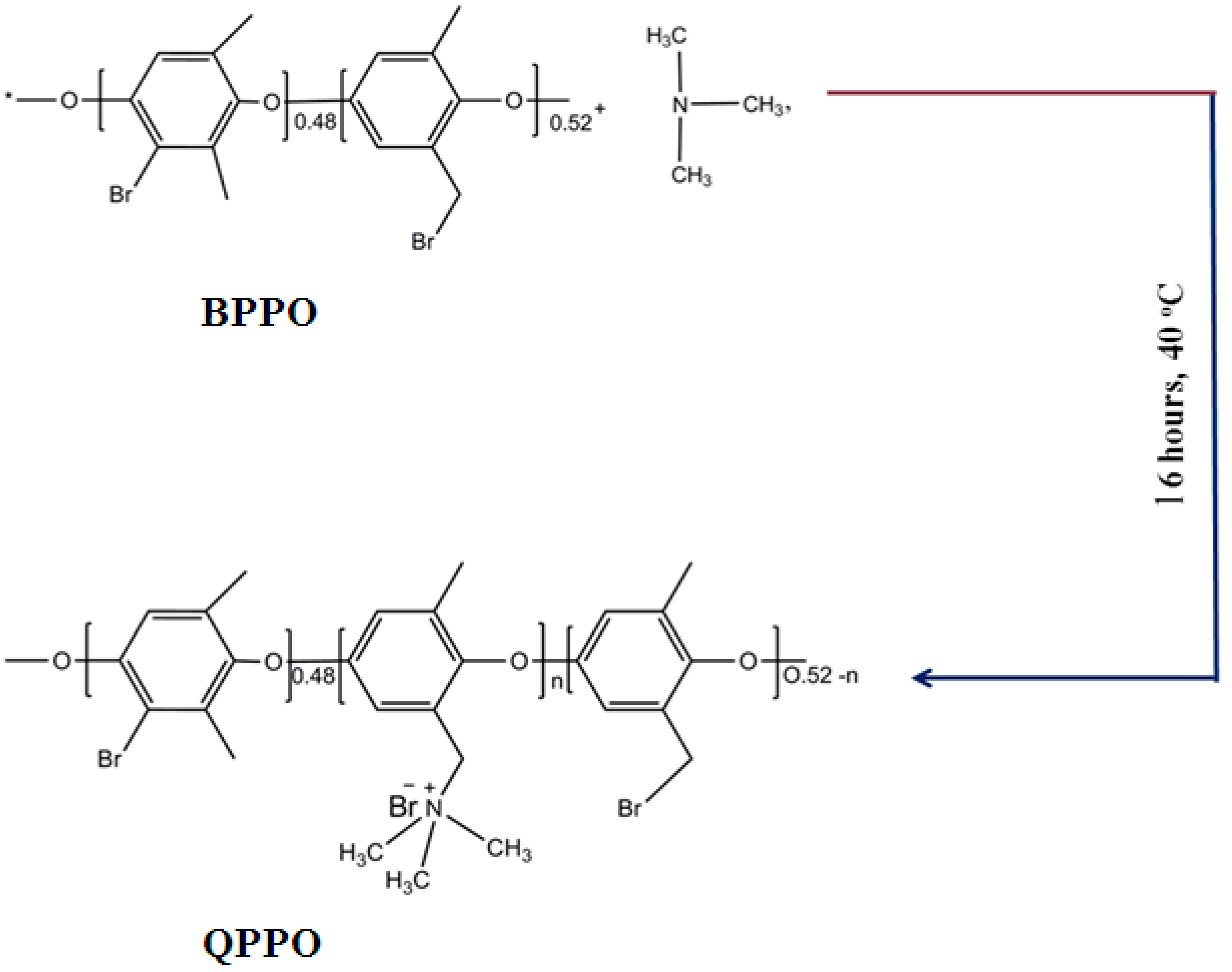
Membranes | Free Full-Text | Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater

Phenol Reduces Nitrite to NO at Copper(II): Role of a Proton-Responsive Outer Coordination Sphere in Phenol Oxidation | Journal of the American Chemical Society
![PDF] Role of 1-methyl-4-phenylpyridinium ion formation and accumulation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity to isolated hepatocytes. | Semantic Scholar PDF] Role of 1-methyl-4-phenylpyridinium ion formation and accumulation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity to isolated hepatocytes. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ed580384da41ea551d55a12dd4fd85b7aa445c8f/4-Figure1-1.png)
PDF] Role of 1-methyl-4-phenylpyridinium ion formation and accumulation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity to isolated hepatocytes. | Semantic Scholar
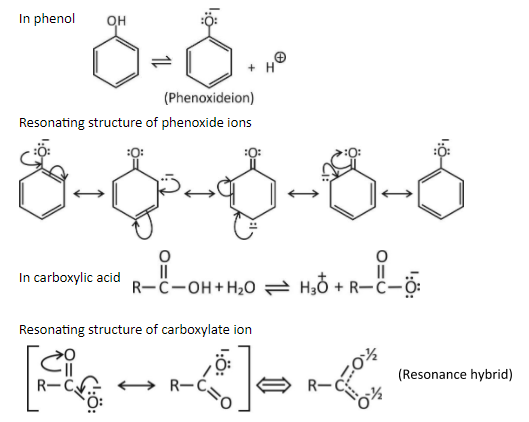
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. why?

Phenyl Oxidation Impacts the Durability of Alkaline Membrane Water Electrolyzer | ACS Applied Materials & Interfaces
Explain why is phenoxide ion more stable than phenol towards electrophilic substitution reaction? - Quora
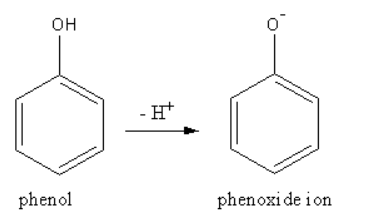
Phenol is acidic because of resonance stabilization of its conjugate base, namely:A. Phenoxide ionB. Epoxide ion C. Benzoate ionD. None of these