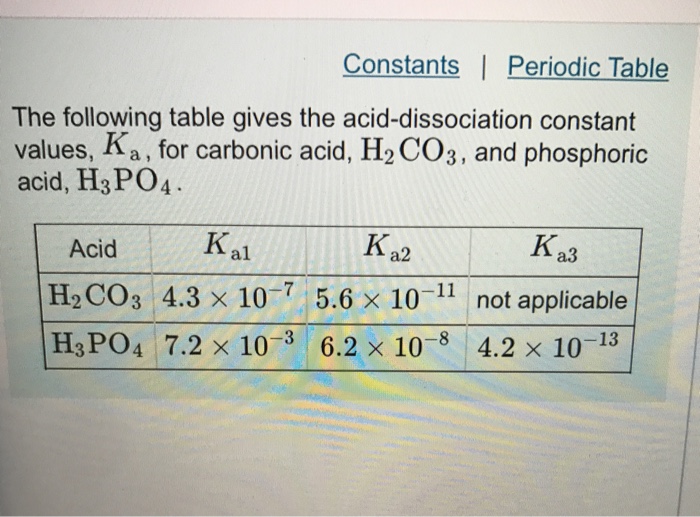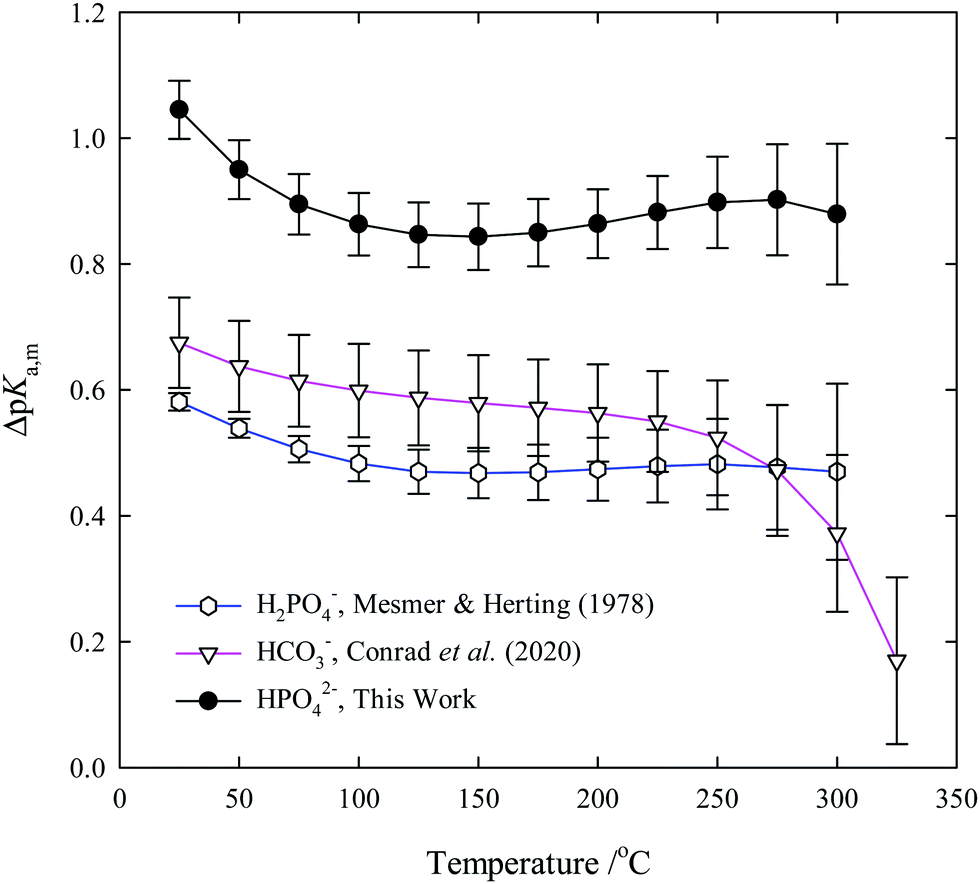
Third dissociation constant of phosphoric acid in H 2 O and D 2 O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire f ... -

Phosphorous acid Acetic acid Phosphoric acid Acid dissociation constant, salt, angle, text, logo png | PNGWing

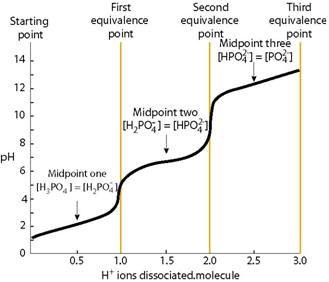
Chemistry Laboratory: Neutralization of a polyprotic acid with a strong base (Key words: Phosphoric acid)

Acid dissociation constants and cytotoxicity test of a series of omega-aminoalkyl phosphates - ScienceDirect
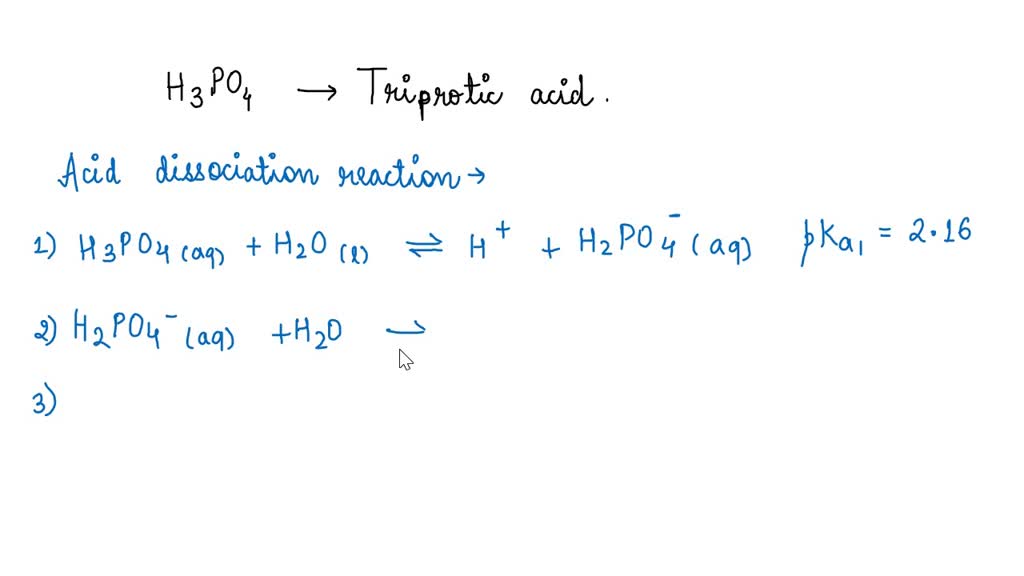
SOLVED: Phosphoric acid, H3PO4 is a triprotic acid. pKa1= 2.16 pKa2=7.21 pKa3=12.32 Write three separate acid dissociation reactions for the three acidic protons. Make sure to indicate which Ka and Kb value
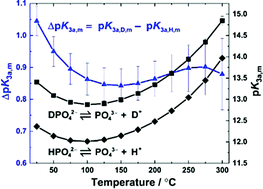
Third dissociation constant of phosphoric acid in H2O and D2O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire flow cell - Physical Chemistry Chemical Physics (RSC Publishing)

OneClass: What is the concentration of phosphate ions in a 2.5 M solution ofphosphoric acid?(The pH o...